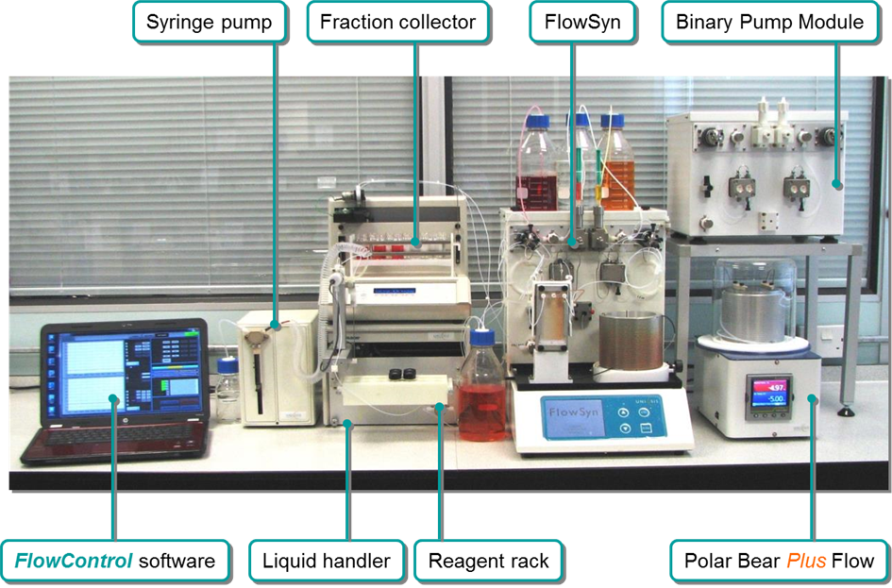
The art of chemical synthesis continues to evolve through innovation in instrumentation, Interchim works with many companies to offer this enabling technology to leading research scientists across Europe.
One such technology is continuous flow chemistry, and in this area, Interchim collaborates with Uniqsis, a market leader in meso scale flow chemistry. This is a complementary technique to batch and microwave for seamless reaction optimisation, synthesis and scale-up from milligrams to 10 Kg per day.
Interchim offers a wide range of flow chemistry products to make this technique accessible to novices while at the same time catering for complex multi-step fully automated reactions sequences for library synthesis.
Flow Chemistry offers several benefits
Faster reactions
- by superheating at elevated pressure, reactions run more quickly (Arrhenius equation)
- batch reactions that take several hours can often be performed in minutes, analogous to microwave
Improved safety
- small reactor volumes minimise risk associated with hazardous intermediates and highly exothermic chemistry
High reproducibility
- precise control of mixing and reaction temperature often difficult in batch chemistry
Scalable
- continuous processing on a mesoscale can deliver 100s g to 10 Kg per day
Solvents
- more choice- superheating
- easier to clean-up – saves time and money
- lowest cost
- best solubility
- least damaging to the environment
To give you an idea on how to minimise risk associated with hazardous intermediates with a small reactor volume, we describe a Curtius Rearrangement carried out with a The FlowSyn™.

Objective:
The Curtius rearrangement is a useful reaction in synthesis that converts carboxylic acids into their corresponding reversed amino derivatives.
However, the reaction requires the formation of potentially explosive acyl azides as the precursor to isocyanates that undergo nucleophilic attack to afford the reaction products. 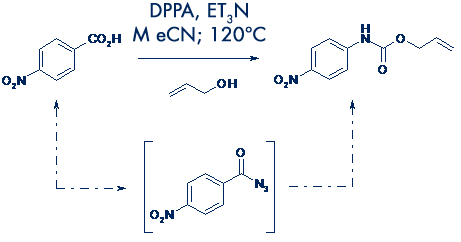 Under conventional ‘batch’ conditions, the scale of the reaction is therefore often limited for safety reasons. This can present a bottleneck in terms of scale-up.
Under conventional ‘batch’ conditions, the scale of the reaction is therefore often limited for safety reasons. This can present a bottleneck in terms of scale-up.
Flow chemistry offers an attractive alternative whereby the acyl azide intermediate is continuously processed through to product, preventing its accumulation.
Method:
System solvent: Acetonitrile.
Stock solution A: 4-Nitrobenzoic acid (925 mg; 5.05 mmol), triethylamine (1.40 mL; 10.0 mmol) and allyl alcohol (1.02 μL; 15.0 mmol) in MeCN (50 mL).
Stock solution B: Diphenylphosphorylazide (DPPA: 1.10 mL; 5.1 mmol) in MeCN (50 mL).
A 100 psi chemically inert fixed back-pressure regulator was fitted and used in all experiments.
Using Automated Experiment Setup
FlowSyn™ is equipped with a program that allows unattended operation and is able to run a flow experiment automatically, stopping and cleaning the instrument when the reaction is complete.
1- FlowSyn™ was fitted with a 14 mL HT PTFE tubing reactor cassette, and the heating unit was tensioned to ensure optimal thermal contact.
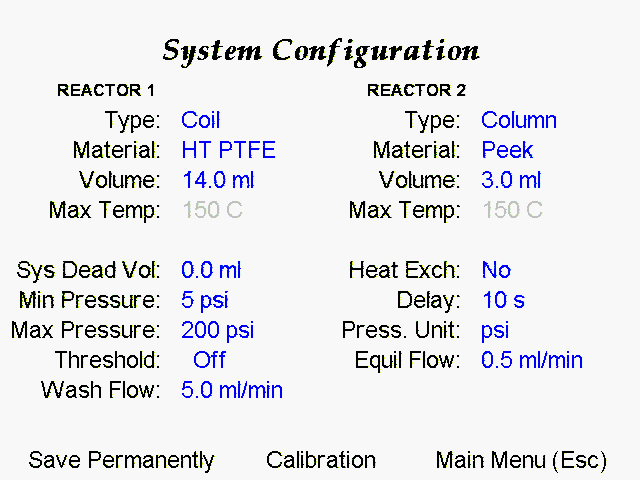
2- A 10 cm x 15 mm Column reactor was filled with a [1:1] mixture of Amberlyst A-21 and Amberlyst H-15 resins, and the ‘Col Vol’ was set to 3.0 mL in the Configuration Screen.

3 – A 100 psi fixed BPR was connected in-line to the outflow from the tubing reactor before the collection valve.

4 – The pumps and inlet lines were primed.
5 – The following flow parameters were entered into the ‘Auto Set Up’ screen.
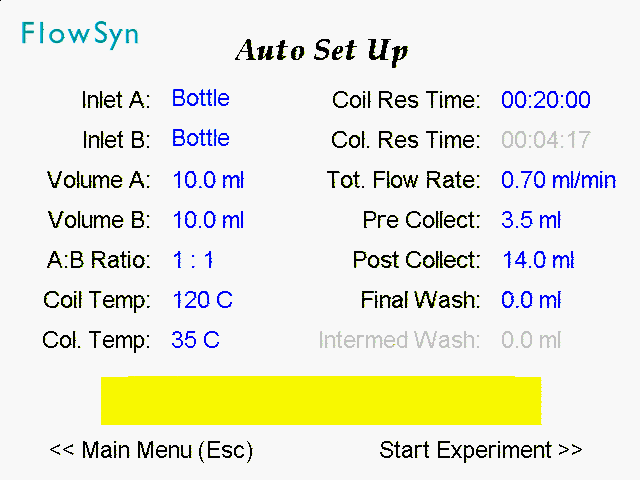
6 – Upon pressing ‘Run Experiment’, FlowSyn™ equilibrates to the set temperature and then runs the flow experiment, before finally cleaning the system by flushing with system solvent (‘Wash’).
7 – The collected product solution was concentrated in vacuo to leave allyl-4-nitrophenyl carbamate as a white solid (198 mg; 88%).
UVLC-MS (ESI +ve): (m/z 223.1 (MH+)); Rt = 3.60 min, >99%;
IR (ATR): 3380 (s), 1730 (s),1685 (m), 1610 (m), 1600 (m), 1545 (s), 1508 (s), 1495 (s), 1320 (s), 1305 (s),1205 (s), 1110 (s), 1050 (s), 945 (s), 850 (s), 765 (s), 750 (s), cm−1.
1H NMR (d3-MeCN, 400 MHz): dH 8.28 (1H, s), 8.15 (2H, d, J = 9.2 Hz), 7.65 (2H, d, J = 9.2 Hz), 5.98 (1H, dt, J = 17.3, 10.2, 5.8 Hz), 5.40 (1H, ddt, J = 17.2, 1.6, 1.6 Hz), 5.30 (1H, ddt, J = 10.8, 1.6, 1.6 Hz), 4.65 (2H, d,J = 5.6 Hz)
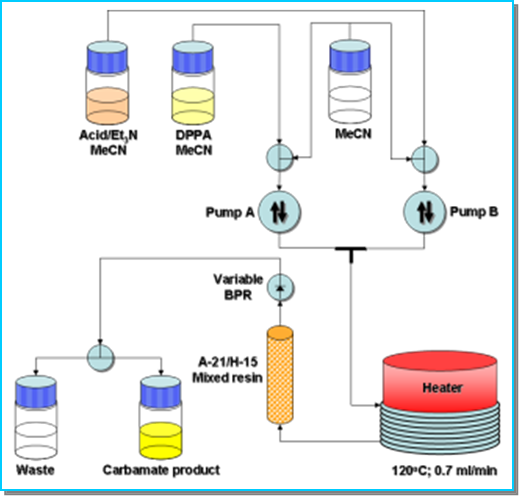
In this specific case flow chemistry is an excellent alternative to push back the limits of the conventional batch chemistry and to overcome a bottleneck in terms of scale-up.
With our range of flow chemistry systems, we offer modular starter systems to fully integrated fully automatic system.
The FlowLab can offer up to 3 reagent channels, 2 reactor stations and has its own software program for single experiments. It also has the option for an inline UV/VIS detector system to see when the reaction has reached steady state.
All of the reagent have been specially designed for flow chemistry and can deliver 10 ml/min or 50 ml/min with the prep heads at up to 100 bar. Reactions can be carried out in the range -85°C to 300°C.