Stationary phase choice:
The choice of sorbent depends on the nature of the compounds to purify => polarity, functional groups.
The retention of compounds is very different depending on the sorbent used.
To avoid stain deformations, silica is generally chosen for the acidic compounds & alumina for the basic compounds.
Non-bonded polar stationary phases: silica, alumina, etc. are materials extremely eager of water.
If kept under open air, they lose their activities by quickly absorbing atmospheric water (50% in less than 3 min). This can lead to completely different separations in between two plates from the same batch that been left at ambient air and carried out at different times.
It is recommended to keep the plates in a desiccator, possibly under vacuum, in the presence of a desiccant.
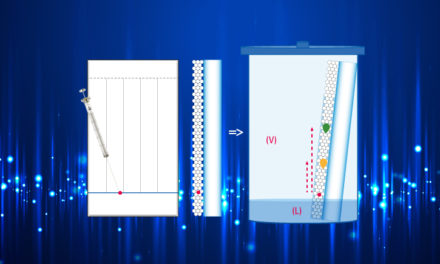
Mobile Phase choice:
What is the ideal distribution of stains on a plate:
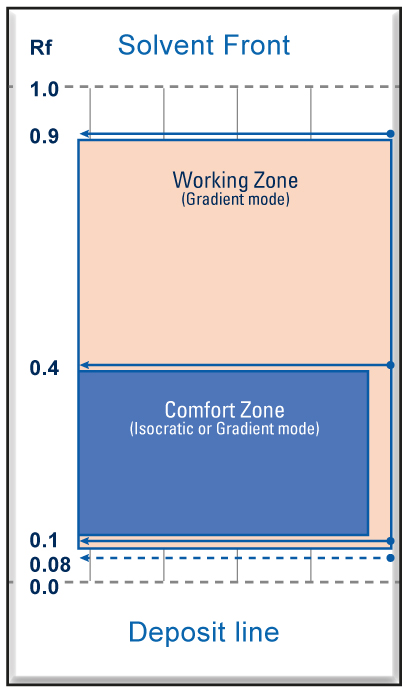
| To get a good location accuracy of the center of the spots and to calculate the Rf it is necessary that they are distributed regularly in the Rf range from 0.08 to 0.9. The best is a spot distribution between 0.1 and 0.4 with a minimal ΔCV. With silica and alumina the more the mobile phase is polar the more the solutes are eluted towards the front of the solvent, towards large values of Rf (Rf ≥ 0.6). Conversely, the more the mobile phase is none-polar, the less the solutes are entrained and the closer they stay to the deposit line with low values of Rf (Rf ≤ 0.1). |
 |
The mobile phase has the following role:
- Dissolution of the sample
- Desorption of the sample from the stationary phase
- Transport of the sample at an acceptable migration distance
In general, the mobile phase must be:
- As simple as possible (maximum 3/4 components)
- None-toxic
- From a chromatographic quality
- Specific to not generate side reactions
- Selected to avoid demixing (vapor pressures, equivalent polarities)
- Having a low viscosity
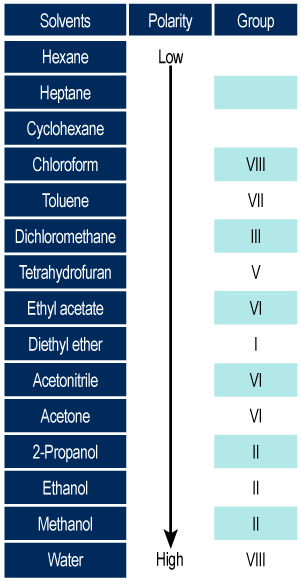
Polarity of mobile phases:
The concept of polarity of the chemical species and the different scales of polarity are described in the purification chapter.
How to control retention:
Two solvents with total missibility parameter values δT , eluent force Ɛ°or polarity P ‘equal or very close will lead, for the same compound, to neighboring or equal retention parameters (even k, or even Rf).
How to change the separation by keeping the retention with same magnitude:
By cons for a pair of solutes with a slightly different polarity, the selectivity (separation of spots) will not be the same for two solvents with identical polarity (δT ou Ɛ° identical or similar) as they express different partial dominant polarity (Partial polarities of solvents must be taken into account).
Eluent strenght on different stationnary phases:ε0 silica = 0.77ε0 alumina |
The classification of solvents according to Trappe is expressed in eluotropic series classified by increasing eluent force:
- Based on the adsorption energy per unit area of the stationary phase
- Depends on the stationary phase
- The classification uses pentane as a reference.
- Eluotropic series on different adsorbents:
| Solvents List | ξ0 Silica Virgin | ξ0 Alumina | ξ0 Silica Diol | ξ0 Silica CN | ξ0 Silica NH2 | ξ0 Silica C18.C4.C8.PH.RPAQ | ξ0 Magnésie | ξ0 Florisil |
| Acetone | 0.470 | 0.560 | 0.141 | 0.470 | 0.470 | 0.325 | 0.291 | |
| Acetonitrile | 0.501 | 0.650 | 0.150 | 0.501 | 0.501 | 0.577 | 0.377 | 0.338 |
| Benzene | 0.246 | 0.319 | 0.074 | 0.246 | 0.246 | 0.185 | 0.166 | |
| Butanol | 0.550 | 0.714 | 0.165 | 0.550 | 0.550 | 0.414 | 0.371 | |
| Carbon tetrachloride | 0.139 | 0.180 | 0.042 | 0.139 | 0.139 | 0.104 | 0.094 | |
| Chloroform | 0.260 | 0.400 | 0.078 | 0.260 | 0.260 | 0.232 | 0.208 | |
| Cyclohexane | 0.030 | 0.0400 | 0.000 | 0.000 | 0.000 | 0.023 | 0.021 | |
| Cyclopentane | 0.000 | 0.05 | 0.000 | 0.000 | 0.000 | 0.029 | 0.026 | |
| 1.2-Dichloroethane | 0.339 | 0.490 | 0.102 | 0.339 | 0.339 | 0.284 | 0.255 | |
| Dichloromethane | 0.323 | 0.420 | 0.097 | 0.323 | 0.323 | 0.244 | 0.218 | |
| Diethylamine | 0.485 | 0.630 | 0.146 | 0.485 | 0.485 | 0.365 | 0.328 | |
| Diethyl ether | 0.385 | 0.380 | 0.115 | 0.385 | 0.385 | 0.220 | 0.198 | |
| Diisopropyl ether | 0.223 | 0.280 | 0.067 | 0.223 | 0.223 | 0.162 | 0.146 | |
| N.N-Dimethylformamide | 0.640 | 0.831 | 0.192 | 0.640 | 0.640 | 0.482 | 0.432 | |
| Dimethyl sulfoxide | 0.470 | 0.620 | 0.141 | 0.470 | 0.470 | 0.360 | 0.322 | |
| Dioxane | 0.490 | 0.560 | 0.147 | 0.490 | 0.490 | 0.325 | 0.291 | |
| Ethanol | 0.677 | 0.879 | 0.203 | 0.677 | 0.677 | 0.510 | 0.457 | |
| Ethyl acetate | 0.380 | 0.580 | 0.114 | 0.380 | 0.380 | 0.336 | 0.302 | |
| Heptane | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Hexane | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.006 | 0.005 | |
| Hexanol | 0.385 | 0.500 | 0.115 | 0.385 | 0.385 | 0.290 | 0.260 | |
| Isooctane | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.006 | 0.005 | |
| Isopropanol | 0.590 | 0.820 | 0.177 | 0.590 | 0.590 | 0.476 | 0.426 | |
| Isopropyl chloride | 0.223 | 0.290 | 0.067 | 0.223 | 0.223 | 0.168 | 0.151 | |
| Methanol | 0.732 | 0.950 | 0.219 | 0.732 | 0.732 | 0.450 | 0.551 | 0.494 |
| Methyl acetate | 0.393 | 0.510 | 0.118 | 0.393 | 0.393 | 0.296 | 0.265 | |
| Methyl ethyl ketone | 0.393 | 0.510 | 0.118 | 0.393 | 0.393 | 0.296 | 0.265 | |
| Methyl tert-butyl ether | 0.470 | 0.610 | 0.141 | 0.470 | 0.470 | 0.354 | 0.317 | |
| Pentane | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Petroleum ether | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.006 | 0.005 | |
| Propanol | 0.631 | 0.819 | 0.189 | 0.631 | 0.631 | 0.475 | 0.426 | |
| Pyridine | 0.550 | 0.714 | 0.165 | 0.550 | 0.550 | 0.414 | 0.371 | |
| Tetrahydrofuran | 0.346 | 0.449 | 0.104 | 0.346 | 0.346 | 0.726 | 0.261 | 0.234 |
| Toluene | 0.223 | 0.290 | 0.067 | 0.223 | 0.223 | 0.168 | 0.151 |
Solvent selectivity according to Snyder chart:
The retention of compounds is different according to selectivity group.
Based on the chemical structure of the compound choose, a solvent which interacts with compounds and is from different solvent group.
If the resolution is not achieved then try an alternative eluting solvent.
Based on the solvent selectivity, the choice of the solvent will be different for purification with normal phase, reversed phase or other technique.
Solvent Selectivity Group Triangle
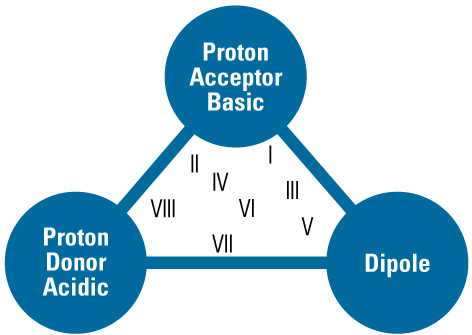
| Solvent Property | Example Solvent | Interacting Compounds |
| Dipole | Dichloromethane | Corbonyl, nitriles, sulphonates, amides |
| Proton acceptor | Amines, ammonia | Alcohols, acids, phenols |
| Proton donor | Alcohols, chloroform | Amines, sulphonamides |
LR Snyder, J Chromatogr. 92 (1974) 223
LR Snyder, J Chromatogr. Sci 16 (1978) 223
Know more:
- Contact us: interfine@interchim.com
- Visit our website: www.interchim.com
- Discover all our purification systems
- Revolutionize your TLC using our App “TLC to Flash & Prep Chromatography”