TLC, compared to liquid chromatography on column, shows differences:
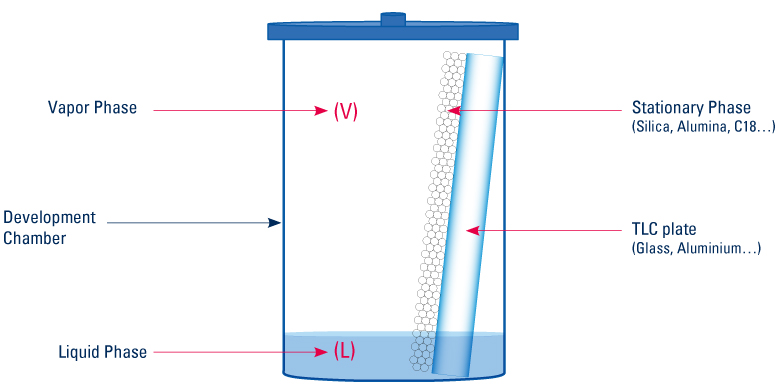
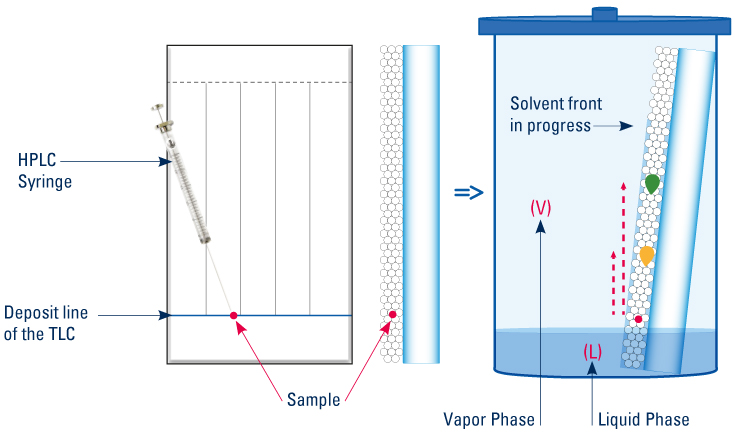
The mobile phase discovers the stationary phase while moving through the plate. The thin layer is not in equilibrium with the elution solvent, as it is the case in a column, but with the solvent vapors contained in the development chamber.
To set up a TLC analysis, 4 parameters have to be considered.
| Sample | ||
| Vapor Phase |  |
Liquid Phase |
| Stationary Phase |
TLC is a chromatography where each of the solutes remains the same time in contact with the mobile and the stationary phases. They travel different migration distances according to their interactions with the phases, whereas in an HPLC column, solutes go through the same total distance. They express a different residence time.
In TLC, the retention of each of the solutes is then characterized by the frontal ratio Rf, whereas on column it is characterized by the retention factor k.
(In the case of a preparative column, the volume of retention, of the mobile phase required to elute the solute, is a considered value.).
Interactions description :
Specificity of the TLC related to evaporation phenomena
| 1: At the liquid (L) – vapor (V) balance, the mobile phase and the vapour phase compositions are not similar because the vapour pressure of the solvents used are generally not the same. At the liquid (L) – vapor (V) balance, according to the composition of the development phase and the respective vapor pressure of its components, the composition of the vapor phase is not the same as the development phase. |
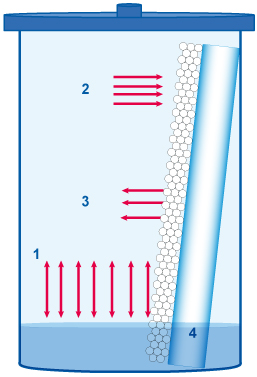 |
2: The dry stationary phase equilibrates with the vapor phase (V) (adsorption saturation). The vapors of polar solvents are much more adsorbed than those of apolar solvents. The composition of the adsorbed phase is different from those of the vapor phase (V) and the development phase (L).
3: During migration the wet stationary phase is re-equilibrated with the vapor phase (V). This concerns the less polar solvents and the more volatile of the migrating liquid.
4: During the migration the components of the mobile phase can be separated by the stationary phase which leads to secondary fronts.
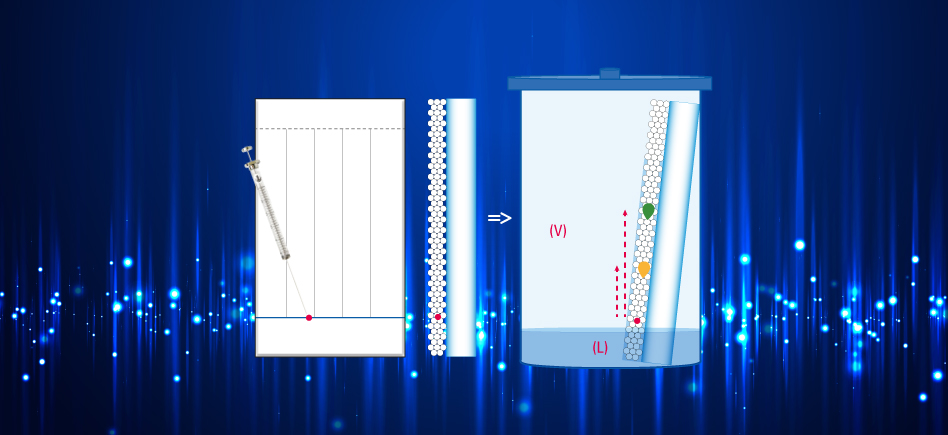
The sample is dropped off, with a capillary, on the deposit line of the TLC plate which is then immersed in the tank containing the mobile phase. This one ascends through the stationary phase by capillarity carrying each compound which moves at its own velocity behind the solvent front according to its affinity for the stationary and the mobile phases.
In TLC, the “retention factor” (Rf) is defined by the ratio of the distance traveled by the analyte (da) over the distance traveled by the solvent front (dS).
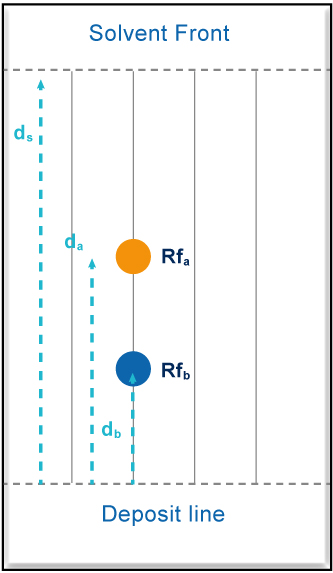 |
Rfa = da / dS Rfb = db / dS In practice, it is necessary to reason in the amount of mobile phase necessary to use to elute the solute out of the column. To take into account there different geometries, this retention volume is expressed relative to the void volume of the column used. It is a dimensionless number identified by the acronym CV (also called Vs) Vsa = CVa = 1/Rfa = 1 + ka Vsb = CVb = 1/Rfb = 1 + kb => ΔCV = CVb – CVa There is a mathematical relation between CV and the retention factor k in liquid HPLC => k = Ktr x(1/Rf – 1) et avec Ktr = cste = 1 => Δk = Ktr x[(1/Rfb – 1) – (1/Rfa – 1)] => CV = Δk |
In practice, it is necessary to control the experimental conditions to obtain reproducible TLC analysis.
Know more:
- Contact us: interfine@interchim.com
- Visit our website: www.interchim.com
- Discover all our purification systems
- Revolutionize your TLC using our App “TLC to Flash & Prep Chromatography”