Solubility
Purpose:
- understand how to choose the solvent that completely solubilizes a sample
In a simplified manner in the majority of situations, two non-electrolyte substances are totally miscible one in the other if: - they have roughly the same molecular size and polarity.
That means that the ratio between the energies of molecular interactions and the molar volume of the two substances is similar.
From the theoretical point of view this has been modeled by Hildebrand, from which it emerges from the studies that: - their total solubility parameters δT must be approximately identical (+/- 2)
- the nature of their main partial solubility parameter (partial dispersion solubility parameter δd or dipolar δp or hydrogen bonds δH must be identical.
In contrast, two solutes with very different total solubility parameters (Δ δT > 3) separate into two distinct phases (demixing). However, in each phase, low concentrations of the other component of the biphasic system are found.
Universal solvents are solvents having total solubility parameters between 10 and 12 and each fractional polarity is close to 33%. They are therefore able to solubilize the majority of the products whatever their polarity. They belong to class E and to a lesser extent to class B as defined in the table and figure below.
The table below shows the values of the total solubility (δT) and partial (δd, δp,δH) parameters of solvents and their fractional polarity parameters (fd, fp et fH) with:
fd = (δd / δd + δp + δH) x 100
fp = (δp / δd + δp + δH) x 100
fH = (δH / δd + δp + δH) x 100
(Particular polymer case: PEG is miscible in water because the molecular interaction energy (binding hydrogen) is the same though the molecular volume is very different).
Example of total solubility and partial parameter values of some solvents
| Solvent |
δT * |
δd * | δp * | δH * | fd ** | fp ** | fH ** | Class |
| MTBE | 6.90 | 6.90 | 0.50 | ? | ||||
| Heptane | 7.40 | 7.40 | 0.00 | 0.00 | 100 | 0 | 0 | A |
| Diethylether | 7.62 | 67 | 23 | 10 | A | |||
| Toluene | 8.90 | 8.67 | 1.00 | 2.00 | 74 | 9 | 17 | A |
| Thf | 9.08 | 8.22 | 3.25 | 3.50 | 55 | 22 | 23 | B |
| Ethyl acetate | 9.10 | 7.44 | 4.60 | 2.50 | 51 | 32 | 17 | D |
| Chloroform | 9.21 | 67 | 10 | 23 | A | |||
| Acetone | 9.77 | 7.58 | 5.70 | 2.00 | 50 | 37 | 13 | D |
| Dichloromethane | 9.93 | 8.91 | 3.00 | 3.10 | 59 | 20 | 21 | B |
| Octanol | 10.30 | 53 | 6 | 41 | C | |||
| Acetic acid | 10.35 | 40 | 19 | 41 | C | |||
| Butanol | 11.30 | 7.81 | 2.50 | 7.80 | 43 | 14 | 43 | C |
| Isopropanol | 11.50 | 39 | 17 | 44 | C | |||
| Acetonitrile | 11.75 | 41 | 43 | 16 | D | |||
| Ethanol | 12.92 | 7.73 | 4.00 | 9.70 | 36 | 19 | 45 | C |
| Methanol | 14.30 | 7.42 | 5.50 | 11.20 | 31 | 23 | 46 | C |
| Water | 23.50 | 7.00 | 8.00 | 20.90 | 19 | 22 | 58 | |
| Methylcellosolve | 12.06 | 7.90 | 4.50 | 7.90 | 39 | 22 | 39 | E |
| Dimethylformamide | 12.14 | 8.52 | 6.70 | 5.50 | 41 | 32 | 27 | E |
| Formic acid | 12.15 | 33 | 20 | 47 | C | |||
| Dimethyl sulfoxyde | 12.93 | 37 | 33 | 30 | E |
*Hansen solubility parameters from J.Roire “Les solvants” EREC (Issy les Moulinwaterx) 1989
** Fractional polarity parameters from J.Roire “Les solvants” EREC (Issy les Moulinwaterx) 1989
Depending on their fractional polarity, the solvents are distributed in 6 different areas of the planar space. Thus they can be grouped into 5 distinct classes plus water that is alone in an area of this space:A class corresponds to solvents developing mainly nonspecific interactions (fd si majority > 80%).
B class corresponds to intermediate solvents between the three preceding classes (fd majority with fH and fp close to 20%).
C class corresponds to solvents developing in addition to the dispersion interactions (fd ~ 40%) some interactions by H bond (fH > 40%).
D class corresponds to solvents developing in addition to dispersion interactions (fd ~ 50%) mainly dipolar interactions (fd > 30%).
E class corresponds to solvents developing in the same way (between 30% and 40%) the three types of interactions.
Finally the water which is apart.
This is shown in the following figure where the zones of solvents of classes A, B, C and D are materialized by red circles or ovals; those of class E by a green oval; finally the water is marked by a small blue circle.
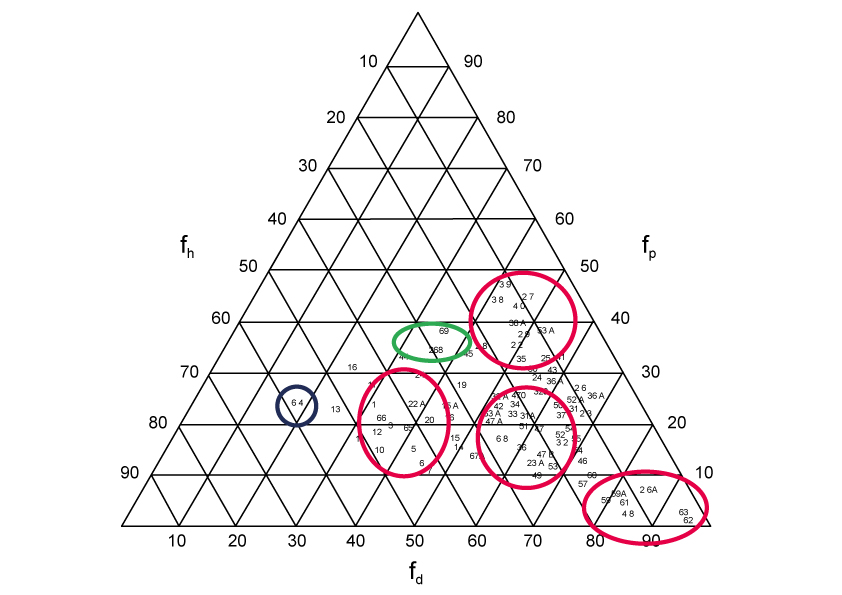
Three-dimensional distribution of some solvents according to their fractional polarity parameter
(from J.Roire “Les solvents” EREC (Issy les Moulinwaterx) 1989)
The total solubility parameter is determined either experimentally or by calculation from the partial solubility parameters. These are obtained from the measurement of the refractive index, the permitivity, the density and the molecular mass of each solvent.
Particular case of the polymers: although their molar volume is very different, PEG is totally miscible in water because their fractional hydrogen binding polarities (δH/δT)2 (or their fractional hydrogen bonding polarity parameter fH ) are close.
Know more:
- Read our article: Purification of a sample: Polarity (part 1)
- Contact us: interchrom@interchim.com
- Visit our website: www.interchim.com
- Discover our product range dedicated to analytical sciences & chromatography
- Discover all our purification systems