Article design and writing: Olivier Mercier, Dominique Desquaires, Didier Charbonneau with the participation of Alain Tchapla and Sylvie Heron, Université Paris-Saclay.
Scientific authorship: Alain Tchapla and Sylvie Heron, Université Paris-Saclay
Purpose:
-
- Determine the chromatographic conditions for the purification of a sample.
- Understand how to choose the solvent which completely solubilizes a sample (for solution or liquid-liquid extraction)
This requires the knowledge of the parameters that lead to the notion of “polarity of molecules”. The polarity is the molecular property that allows to evaluate how and with which intensity one molecule attracts another (molecular interactions), and thus to choose the solvent of a sample but also the chromatographic conditions for the purification.
Polarity
Purpose:
- Predicting the polarity of a substance from its molecular structure.
The polarity of an organic molecule is the property that allows either to predict or to evaluate the nature and the strength of the molecular interactions occurring between two molecules whether they are identical (pure material) or different (in mixture) from one to another.
Polarity is the consequence of the development, accessibility and intensity of the totality of the partial electrical charges developing on the surface of organic molecules.
Under which molecular conditions do partial electrical charges develop between the covalently bonded atoms?
Fisrt, we have to define the molecular structure of the analytes, and in which molecular conditions of partial electrical charges develop themselves at the surface of a molecule.
Organics molecules mainly contain: hydrocarbon skeleton at which functionalized groups may be added. This corresponds to two kinds of atoms
- Major atoms (C, H)
- Heteroatoms (O, N, S, P, Halogens)
When 2 atoms are covalently linked, their relative electronic attraction of bonding electrons leads to equal (symmetrical molecular bond) or unequal arrangement of partial charges in the molecule. The charge distribution is marked with the symbols δ+ and δ–. Thus, for each covalent bond a dipole is associated, to which correspond a dipolar moment. The vector sum of all dipolar moments leads to the molecular dipolar moment of a given structure.
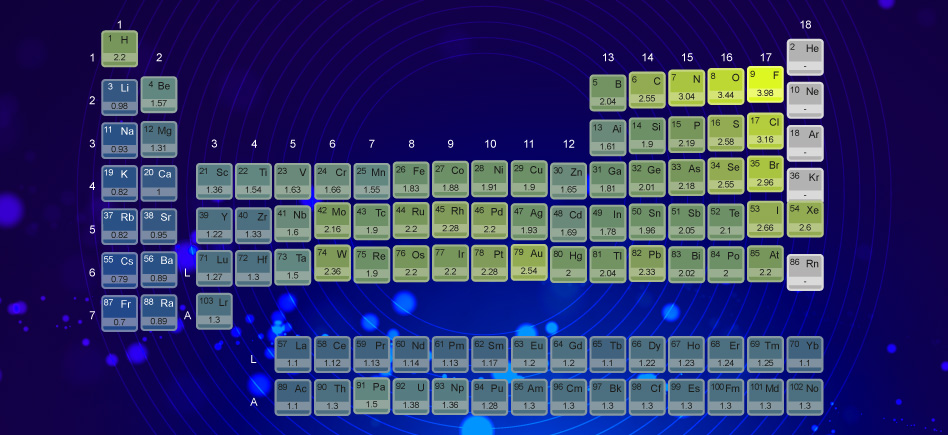
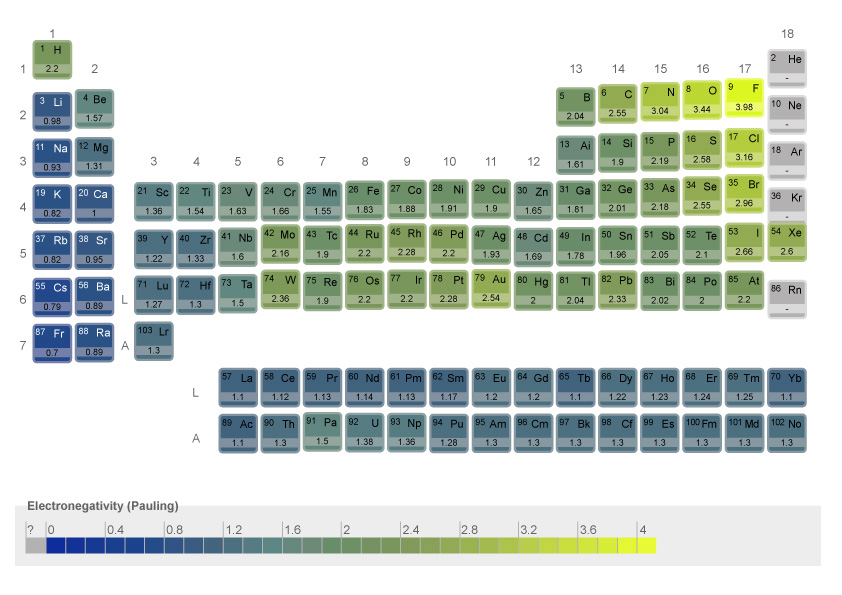
Predict the nature of partial electric charges which respectively appear on two covalently bonded atoms refers to Pauling electronegativity scale.
The electronegativity (χ) is defined as the attraction power of an atom for an electron. Due to their specific structure, the atomic nucleus and electronic cloud of atoms generate their electronegativity.
In the periodic table of elements, the electronegativity, increases from left to right and from bottom to top.
There are four different ways for developing partial electric charges on a covalent bond. They lead to four different kinds of dipoles.
a) Instantaneous dipole δd
For linked atoms having the same electronegativity, binding electrons move quickly from one of the linked atom to the other and none unbalanced permanent charge occurs. However, instantaneously asymmetrical partial electric distribution appears which is immediately reversed.
The emergence of two reciprocal partial electric charges on each of the bonded atoms forms an instantaneous dipole which changes direction at every instant. In the case of organic molecules, these instantaneous partial charges develop on each C-C bond; more particularly in the case of alkanes, but also along the hydrocarbon chains of the functionalized molecules. Their intermolecular attraction potential is characterized by the partial dispersion solubility parameter δd which will be defined in the next chapter: solubility.
b) Permanent dipole δp
For two different bonded atoms (C-O; C=O; C-N; C-X…), due to their different electronegativity, a permanent unequal repartition of partial electrical charge is created. However, the global environment in which the heteroatom is inserted must, be taken into account: it must not be located in a system of symmetrical links with respect to a center (for example, CO2 does not have a permanent dipole because it is a linear molecular structure whereas H2O or Et-O-Et develop a permanent dipole because they are non-linear molecular structures). In the midst of the classes of molecules corresponding to this property are the ketones, the esters, the halogenated compounds, the tertiary amides, the nitriles…
Their potential intermolecular attraction is characterized by the partial dipolar parameters δp.
c) Peculiar case: notion of dipole causing hydrogen bonding
These interactions appear when in a structure OH, NH or SH functions are present. The very large difference in electronegativity between the heteroatom (O, N or S) and hydrogen leads to the creation of a permanent dipole. The power of attraction between the charge δ+ on the hydrogen and the charge δ– on the heteroatom is so strong that it leads to the creation of an intermolecular interaction between the H of a molecule with the heteroatom of the neighboring molecule to form the so-called hydrogen bond.Their intermolecular attraction potential is characterized by the partial hydrogen bond solubility parameter δH, which will be defined in the following chapter: solubilit. Among the classes of molecules corresponding to this property are the alcohols, phenols, carboxylic acids, amines I and II, amides I and II, thiol…
d) Induced dipole δd
When a polar molecule showing a permanent dipole is close to a neutral but polarizable molecule, its electric field is creating an induced dipole moment on this molecule leading to an unequal repartition of the electric charge.
This case occurs for molecules with multiple bonds C = C and C Ξ C or a carbon bonded to a large polarizable heteroatom, for example C-I in interaction with a polar molecule.
Amidst the molecule classes corresponding to this property, we can find aromatic acetylenic or ethylenic unfunctionalized hydrocarbons.
Their potential molecular attraction is integrated in the partial solubility parameter of dispersion δd.
Total resulting dipole δT
The total polarity of a molecule is the sum of all the contributions of the partial polarities described above. (vector sum of all the dipole moments of each bond of a molecule) Their intermolecular attraction potential is included in the total solubility parameter δT , which will be defined in the next section: solubility.
It should be noted that, according to the small difference in electronegativity between hydrogen and carbon, each C-H bond has a very weak dipole moment. In space, thanks to the free rotation around the C-C bonds this effect is canceled out overall. But, this implies that instantly, the longer the chain is, the greater the influence of these instantaneous intermolecular attractions becomes strong. This makes possible to understand that the alkanes, non-polar solutes, can possess a permanent molecular attraction power, because they are liquid at atmospheric pressure from 5 carbons of linear chain up to 15 carbons and then solids above 16 carbons of chain.
For a good interpretation of physical properties of the molecular species, it is necessary to take into account 2 types of interactions:
- Van Der Waals interactions, rely on molecule polarity and polarizability.
- The interactions linked to the intermolecular and some intra molecular hydrogen binding.
| Interaction | Mecanism | Molecules types | Involved compounds |
| Van der Waals | Debye + Keesom + London | Apolar molecules | Alkyl chains, aromatic rings |
| Dipolar Debye | Permanent dipole – instantaneous dipole | Polar molecules and any molecules | |
| Dipolar Keesom | Permanent dipole – permanent dipole | 2 polar molecules | |
| Dipolar London | Instantaneous dipole – instantaneous dipole | 2 any molecules | |
| Hydrogen binding | Proton acceptor – proton donor | Alcohols, amines, acids |
Know more:
- Read our article: Purification of a sample: Solubility (part 2)
- Contact us: interchrom@interchim.com
- Visit our website: www.interchim.com
- Discover our product range dedicated to analytical sciences & chromatography
- Discover all our purification systems