Flow chemistry : Introduction to this technique
Flow chemistry is a rapidly emerging technology that has recently gained a place alongside conventional batch and microwave-assisted organic synthesis (MAOS) as a complementary technique for molecule makers.
This alternative strategy reduces cycle times and impact the whole of the drug discovery process from early stage R&D to the eventual successful production of active pharmaceutical ingredients (APIs).
Flow Chemistry pushes back the limits of the classic chemistry by :
- High reproducibility and efficient mixing
- An efficient T°C control
- Accelerated reactions runs
- Scalable
- Reduced the costs
- Improved Safety
Interchim offers an innovative product range of flow chemitry systems developped by UniQsis.
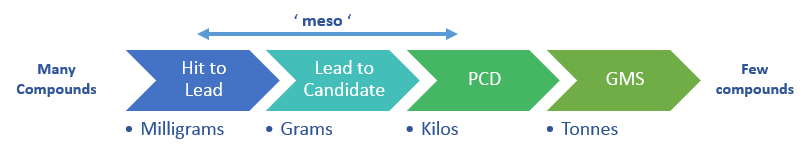
This compact integrated or modular meso-scale continuous range proposed by Interchim can be used for reaction profiling and optimisation, particularly prior to scale up.
It is an ideal research tool, particularly for academic research laboratories and major pharmaceutical companies allowing to achieve applications like hydrogenation, nitration, bromination, metalation, Fischer Indole, Curtius, Suzuki Miyaura, Hantzsch and Newman Kwart chemistries.
The product range:
Configuration possible
FlowLab
Characteristic features of the flow chemistry systems
Highly reproducible and efficient mixing
A particularly appealing approach invokes the use of long, narrow-bore tubing reactors. These can be coupled with either static or dynamic mixers to promote turbulent mixing, but alternatively, where the tubing internal diameter is less than the order of 1 mm (and the Reynolds Number is small), highly reproducible and efficient mixing may be achieved simply by diffusion between contacting laminar flow streams.
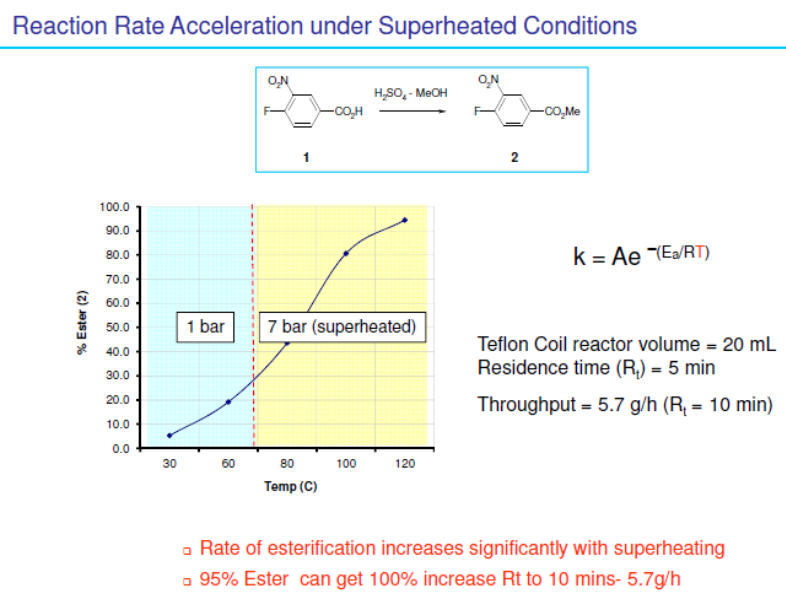
Accelerated reactions run under superheated conditions
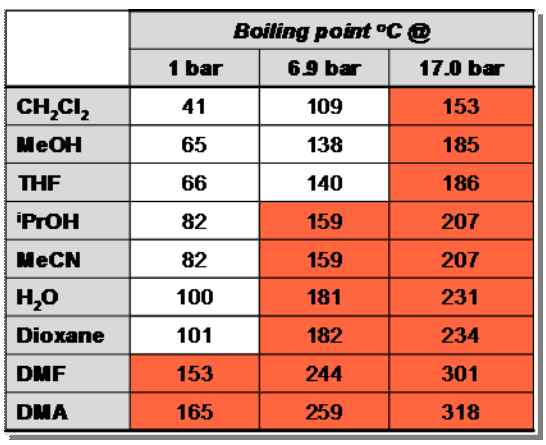
In batch, maximum temperature of the reaction medium is limited by the boiling point of the solvent, itself linked to the atmospheric pressure.
But with the flow chemistry systems , it is possible to superheat the solvents by varying the pressure. The limit being the maximum pressure of the system used, is 100 Bar for the PTFE version, and Stainless Steel 316.
For example, with the FlowSyn version PTFE and Stainless Steel 316, water boils at
200 ° C at 15 bar and dichloromethane at about 100 ° C to 7 bar.
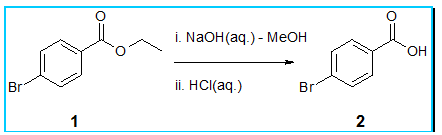
Even simple reactions can benefit from superheating effects. For example, basic hydrolysis of ethyl 4-bromobenzoate 1 (image above) or conversely the acid catalysed esterification of 4-bromobenzoic acid 2 (image below) are both reactions that are significantly accelerated under superheated conditions.
Chemistry performed with microwave systems can also achieved in high T ° C but it is less effective than flow chemistry because of the electromagnetic field of a microwave which is not always homogeneous.
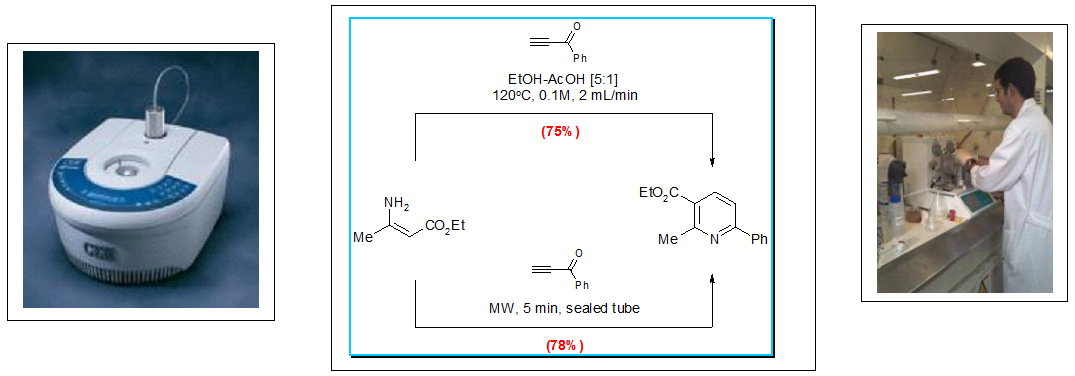
Mark Bagley’s group in Cardiff, for example, have shown that the Bohlmann Rahtz pyridine synthesis performed in an electrically heated flow-through device works just as effectively as when it runs on a small scale in a batch microwave reactor (image below).
Scale-up
Our flow chemistry systems can achieve a scale-up without the optimisation phase while keeping the same operating conditions.
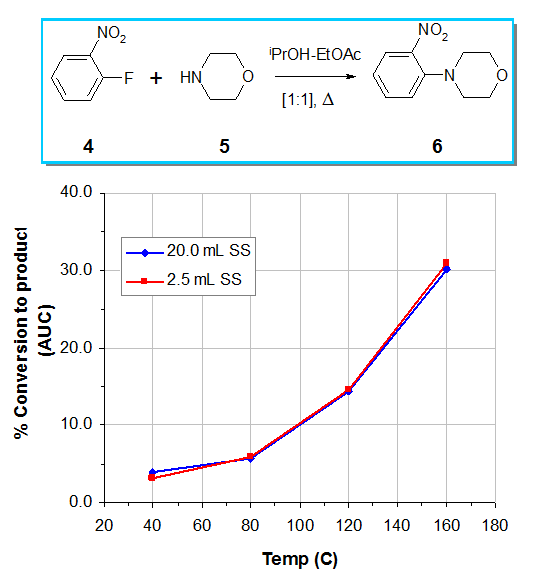
The figure below shows the reaction profile of a simple SNAr reaction when performed under identical conditions in both a 2.5 mL and a 20 mL tubing reactor (identical ids in each case). Even if the size of the loop 20 ml is 8 times greater than the 2.5ml, the profiles of each reaction , remain identical.
Improved Safety
The ability to limit the quantity of a dangerous or unstable reaction intermediate that may be present at any time by invoking continuous processing in a flow-through reactor presents an opportunity to safely perform such reactions on a larger scale.
For example:
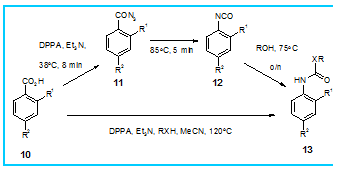
Steve Ley’s group at Cambridgee and scientists within Pfizerf have developed a flow-through procedure for the synthetically useful Curtius rearrangement that is both scalable and safe
The potentially explosive acyl azide intermediate is continuously formed in a limited quantity and then encouraged to undergo a controlled rearrangement to the more stable isocyanate which, in the presence of a suitable nucleophile is converted into the final product (picture above).
The innovative, modular or integrated flow chemistry systems offered by Interchim are easy and intuitive to use. They are an essential research tool for many chemists in the world.
Know more :
- Find all our flow chemistry systems on our web site
- Do not hesitate to contact us for more information on our web site or our various social networks (Facebook, Twitter, Forum).