PhotoRedOx Setup
Interest in photochemistry has been growing exponentially in recent years. Numerous new applications using visible-light photoredox catalysis have been discovered. These catalytic systems can perform many types of bond formations using various substrates which are valuable new tools for synthetic chemists.
However photoredox chemistry setup necessitates to the use of a light source (blue light) and apparatus that are not standard yet in an organic chemistry laboratory. Many chemists have made their own setup and tried to reproduce literature chemistry with more or less success. As a result the implementation of photoredox chemistry is slow and organic chemists are still hesitant to try these important new tools. Therefore, the need for a simple and robust device to perform visible-light photoredox catalysis has become increasingly important.
EvoluChem™ PhotoRedOx Box
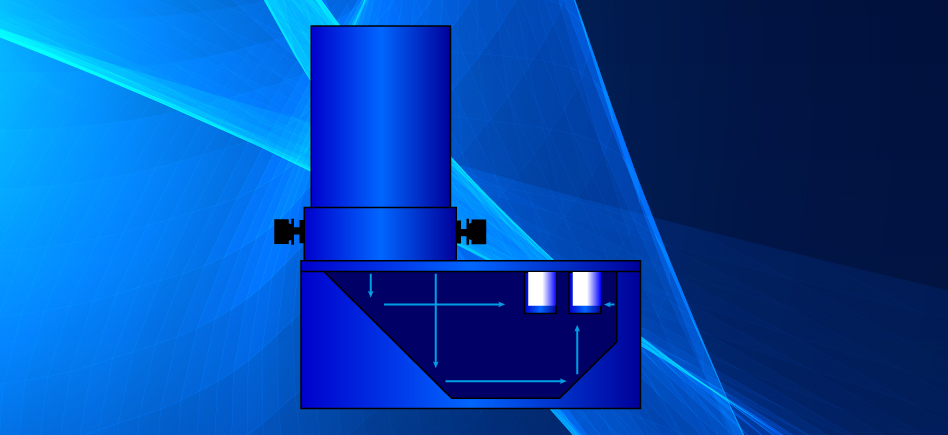
The EvoluChem™ PhotoRedOx Box was designed with one main objective: To allow any chemist to easily perform multiple photoredox reactions in a reproducible environment. Our photochemistry device provide an even light distribution to all reaction samples allowing consistent and reproducible reactions. A cooling fan allows even temperature distribution and keeps the chamber near room temperature during long reaction runs. The device easily fits on standard stir plates, allowing for consistent stirring. Sample holders are compatible with vials ranging from 0.3 ml to 20 ml vials.
Unique Design
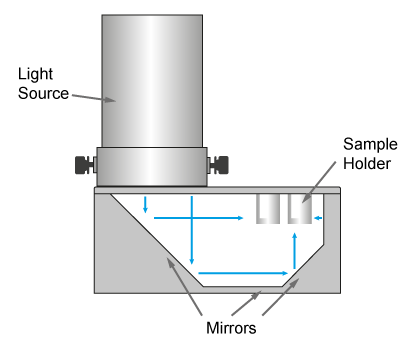 The PhotoRedOx Box is using a unique geometry of mirrors to irradiate multiple samples simulatanously for parallel chemistry setup while limiting the thermal effect of the light source. This design results into a compact and efficient photoredox device which can be easely set on any standard stir plate.
The PhotoRedOx Box is using a unique geometry of mirrors to irradiate multiple samples simulatanously for parallel chemistry setup while limiting the thermal effect of the light source. This design results into a compact and efficient photoredox device which can be easely set on any standard stir plate.
The removable lamp adapter allows easy switching from the standard kessil™ blue 34W LED lamp to many other light sources.
Fit multiple vial formats
 Organic chemists needs to be able to use different reaction vial sizes depending on the scale and the number of the reaction to be performed. The PhotoRedOx Box can virtually fit any type of vials including 0.3ml crimped vials (6 x 32mm), 2ml HPLC vials (12 x 32mm), 1DRAM (15 x 45mm), Microwave vial 2-5mL (17 x 83mm), 2DRAM (17 x 60mm) and 20ml scintillation vials (28 x 61mm).
Organic chemists needs to be able to use different reaction vial sizes depending on the scale and the number of the reaction to be performed. The PhotoRedOx Box can virtually fit any type of vials including 0.3ml crimped vials (6 x 32mm), 2ml HPLC vials (12 x 32mm), 1DRAM (15 x 45mm), Microwave vial 2-5mL (17 x 83mm), 2DRAM (17 x 60mm) and 20ml scintillation vials (28 x 61mm).
This feature allows quick and consistent scale up from screen reactions to larger scale with preset sample positions removing the guess work on sample placement distance from the light source. When using 0.3 ml vials, 32 reactions can be performed in parallel in the photochemical device. At 20 ml, two reactions can be run in duplicate.
Available Holders
 |
 |
 |
 |
 |
Reproducibility
With the EvoluChem photomethylation kit, we have demonstrated the reproducibility of both the photomethylation kit and the device. Using a photomethylation of buspirone as test reaction, 16 vials spread through the 0.3 ml vial sample holder for Trial #1 results in 53% (+/-2 %) conversion. See figure. For a second trial with 16 reaction vials we observed an average conversion of 56% (+/-2 %) for the mono-methylated product.
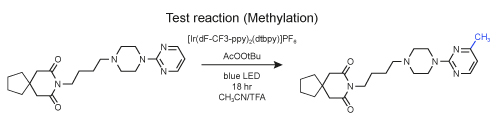
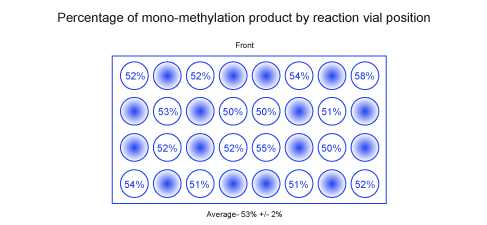
Reaction conditions:
Each reaction vial contains Ir(dF-CF3-ppy)2(dtbpy)[PF6] (0.1 μmol), tert-butylperacetate solution (12.5 μmol) and a stir bar sealed under inert atmosphere. To each vial was added 50 μl of 0.05 M buspirone solution in 1:1 trifluoroacetic acid/acetonitrile sparged with nitrogen stream. Reaction mixture irradiated with Kessil 34 W blue LED for 18 hr using EvoluChem photochemical device.
PhotoRedOx Flow Reactor
 The common limitation to scaling up photoredox chemistry is due to the low penetration of the light in to the reaction mixture (few mm) which prohibits the use of large reaction vessels. Surface area is key to shorten reaction time. It is possible to significantly increase the surface area by running the reaction in flow. This will decreases the reaction time and allows to be run in continuous mode for scale-up.
The common limitation to scaling up photoredox chemistry is due to the low penetration of the light in to the reaction mixture (few mm) which prohibits the use of large reaction vessels. Surface area is key to shorten reaction time. It is possible to significantly increase the surface area by running the reaction in flow. This will decreases the reaction time and allows to be run in continuous mode for scale-up.
To solve this challenge, we designed a flow reactor that can be used in the PhotoRedOx Box. This flow reactor is using PFA tubing and has volume of 2 ml. Comparing reactions in flow and in batch we observed significant decrease in reaction time.
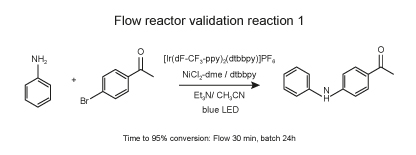
Reaction protocol
In a 4-ml vial equipped with a Teflon septa were weighed NiCl2-dme (1.1 mg, 5 μmol, 0.05 mol %) and dtbbpy (1.3 mg, 5 μmol, 0.05 mol %). 1 ml of dry MeOH was added to the vial and the vial was stirred on an orbital shaker until complete dissolution. The solution was evaporated to dry at room temperature. Then Ir(dF-CF3-ppy)2(dtbpy) (1.1 mg, 1 μmol, 0.05 mol %), and 4-bromoacetophenone (9.95 mg, 100 μmol, 1 equiv.) were added. 1 ml of dry acetonitrile was added followed by Et3N (21 μmol, 300 μmol, 3 equiv.) and aniline (4.65 mg, 100 μmol, 1equiv.). The solution was sparged with nitrogen via submerged needle for 5 minutes.
Several batches of 100 μl of solution were successively injected to the flow reactor placed in EvoluChem PhotoRedOx Box with blue Kessil LED using an injection module (Gilson) and the samples were circulated using a HLPC pump at different flow rates to allow residence time of 5, 10, 15, 20 and 30 min. Reaction completion was monitored by LC-MS using the ratio bromoacetophenone/product.

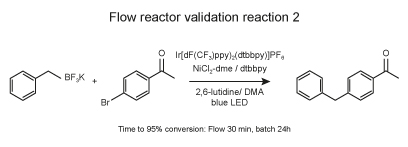
Reaction protocol
In a 4-ml vial equipped with a Teflon septa were weighed NiCl2-dme (1.1 mg, 5 μmol, 0.1 mol %) and dtbbpy (1.3 mg, 5 μmol, 0.1 mol %). 1 ml of dry MeOH was added to the vial and the vial was stirred on an orbital shaker until complete dissolution. The solution was evaporated to dry at room temperature. Then Ir(dF-CF3-ppy)2(dtbpy) (1.1 mg, 1 μmol, 0.1 mol %), and 4-bromoacetophenone (4.98 mg, 50 μmol, 1 equiv.) were added. 1 ml of dry acetonitrile was added followed by 2,6 lutidine (17.5 μmol, 150 μmol, 3 equiv.) and potassium benzyltrifluoroborate (9.90 mg, 50 μmol, 1 equiv.). The solution was sparged with nitrogen via submerged needle for 5 minutes.
Several batches of 100 μl of solution were successively injected to the flow reactor placed in EvoluChem PhotoRedOx Box with blue Kessil LED using an injection module (Gilson) and the samples were circulated using a HLPC pump to allow residence time of 30 min. Reaction completion was monitored by LC-MS using the ratio bromoacetophenone/product.
To know more:
Acces directly to our products dedicated to PhotoRedox on our website.