When purifying peptides, it is very common to have to use TFA as an additive. The use of a mass spectrometer as a detection mode is also common, in order to confirm the masses of the purified products. What happens if we wishe to use both of these conditions at the same time?
TFA binds strongly to the analytes to be purified, resulting in a reduction of the MS signal in the best case, up to total signal suppression, due to the following equilibrium :
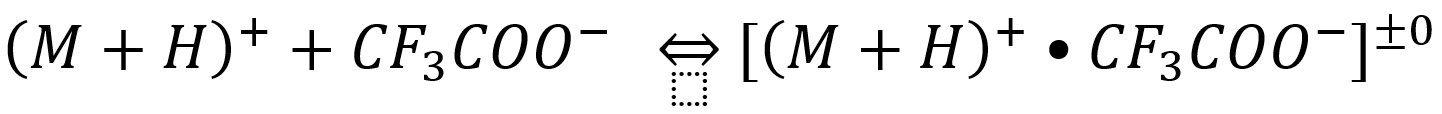
Strong Ion Pairing with TFA-Anion, MS detection is reduced.
It can then be said that these two parameters are incompatible, and that it is therefore necessary to change the purification conditions, for example by replacing TFA with formic acid, by redevelopp a method.
We will demonstrate in this article that a trick can still get a signal, without having to redevelop the method, and thus save time on this step.
Possible solution
Is it possible to change the previously described balance and then invert it to allow detection in mass spectrometry?
Add another acid, such as carbolylic acid is a possible solution.
Both acids (TFA and carboxylic acid) are therefore in competition, and the balance is as follows:

TFA stay in its acidic form, then it can’t interacted with mass detection.
Under these conditions, the first equilibrium, given in the introduction, is then shifted in favour of the form (M+H)+ + CF3C00–, because the carboxylic acid reduces the presence of TFA in the form CF3C00–, detection is then possible!
Application
Lets see if this works with the following application:
Process:
Solvents:
Water + 0.1%TFA
ACN + 0.1%TFA
Gradient: from 5 to 40% ACN in 45min
Samples:
 |
 |
 |
Concentration: 20mg/mL
Injected quantity: 150µL
Column: PFB-15C18N-F0025
Detection:
UV 254nm
MS XIC 1: 235-241m/z
MS XIC 2: 377-383m/z
MS XIC 3: 571-577m/z
TIC 220-1200m/z
Instrument:
puriFlash®5.250 + MS Interface + MS Detector

Scheme:
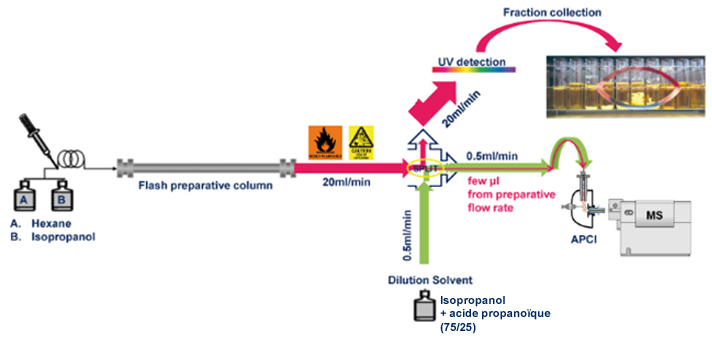
Results:
UV Chromatogram
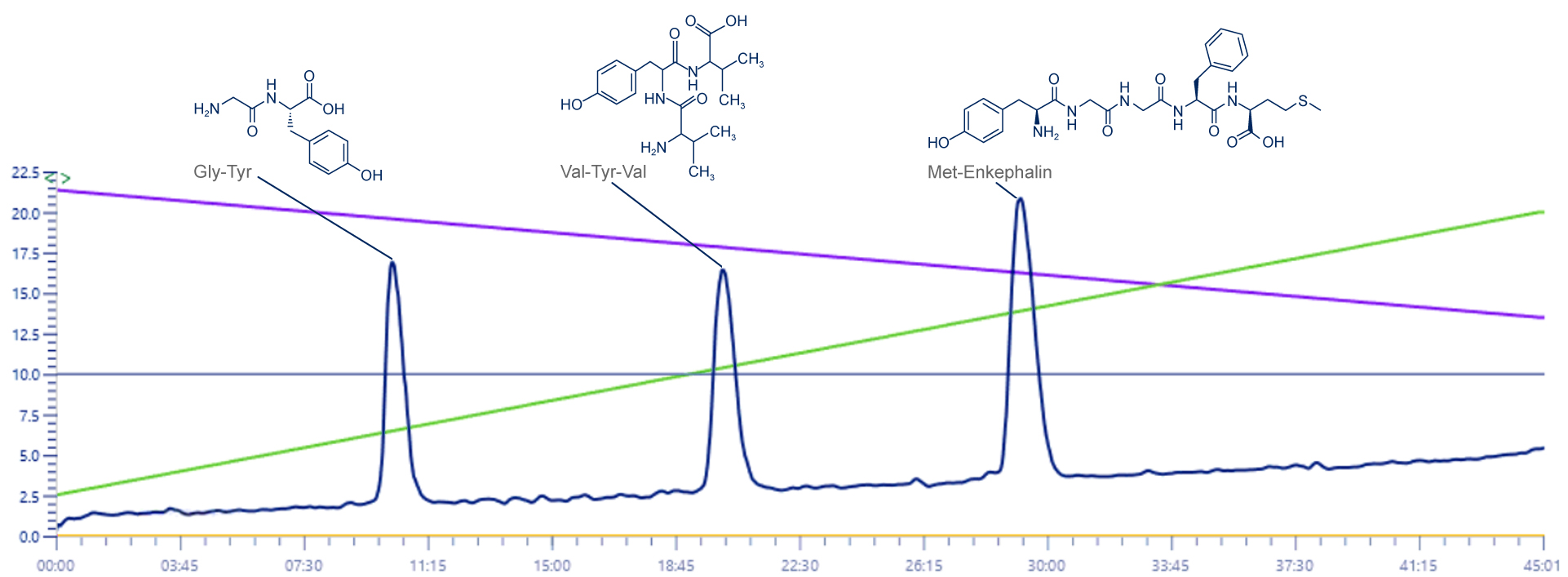 This signal don’t show any modification, it is shown only to provide details…
This signal don’t show any modification, it is shown only to provide details…
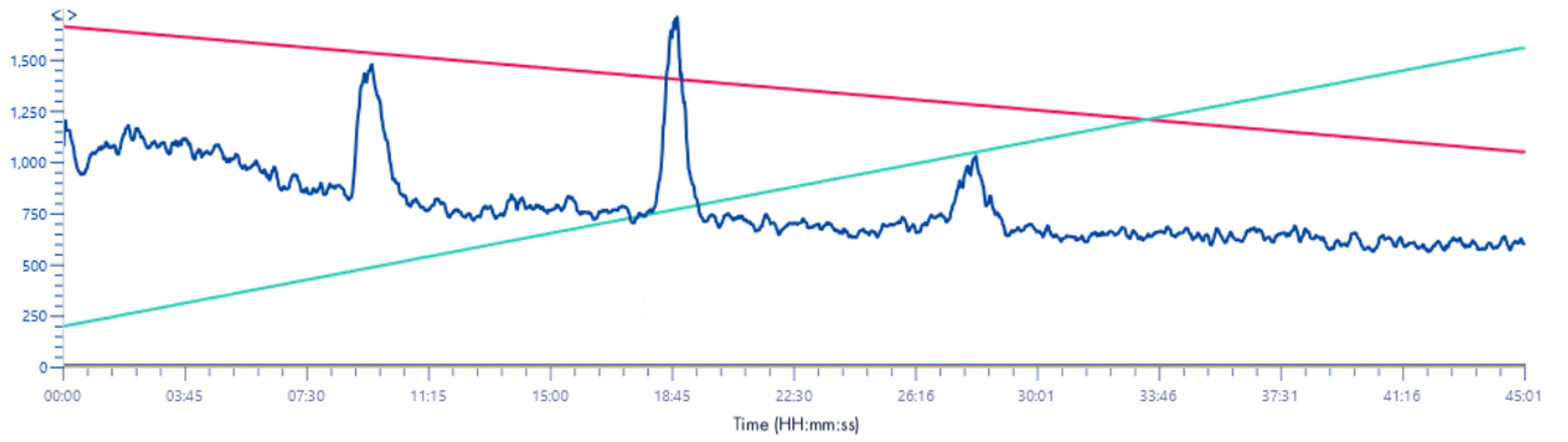
XIC signal 235-241m/z
Solvent make-up: Isopropanol
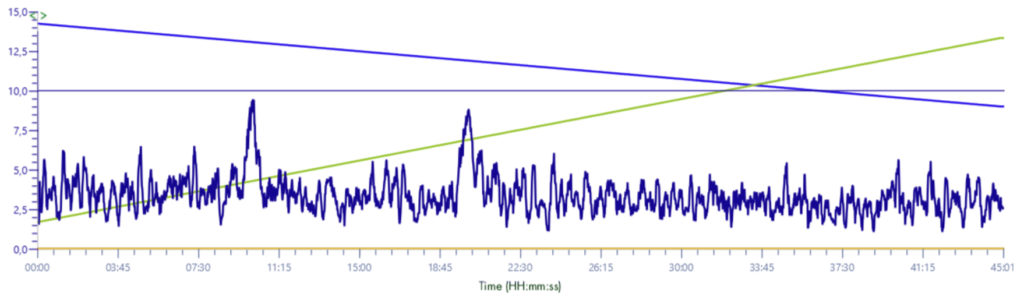
Solvent make-up: Isopropanol + 25% propanoic acid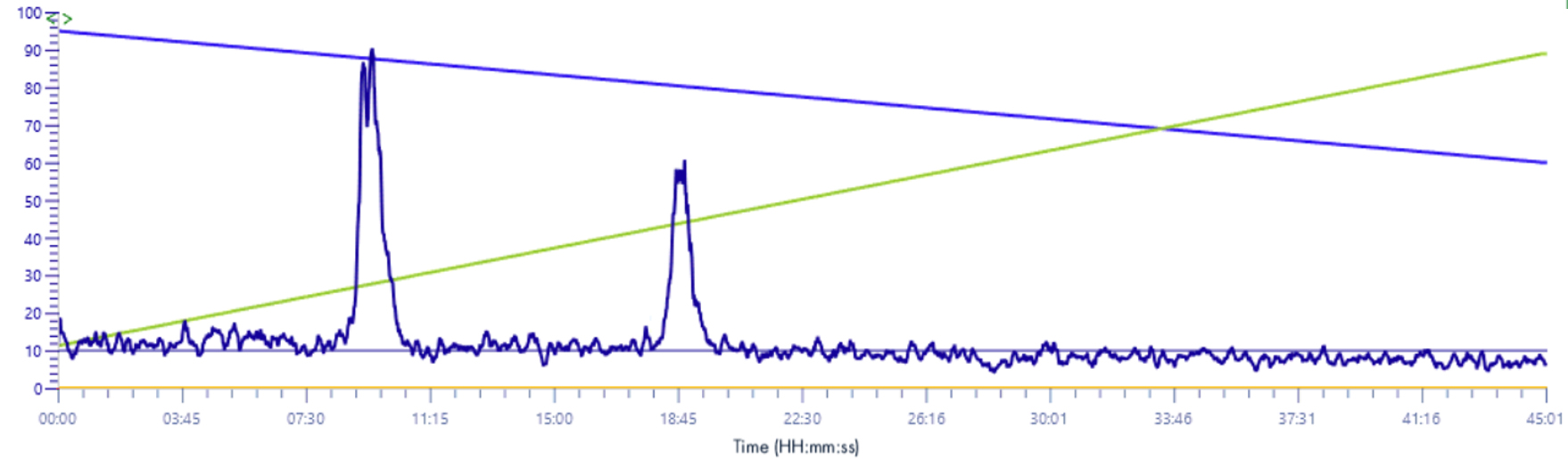
The difference between the signals is important, without this solution the signal is almost absorbed by the background noise.
With this solution the signal gains in intensity and is multiplied by 10.
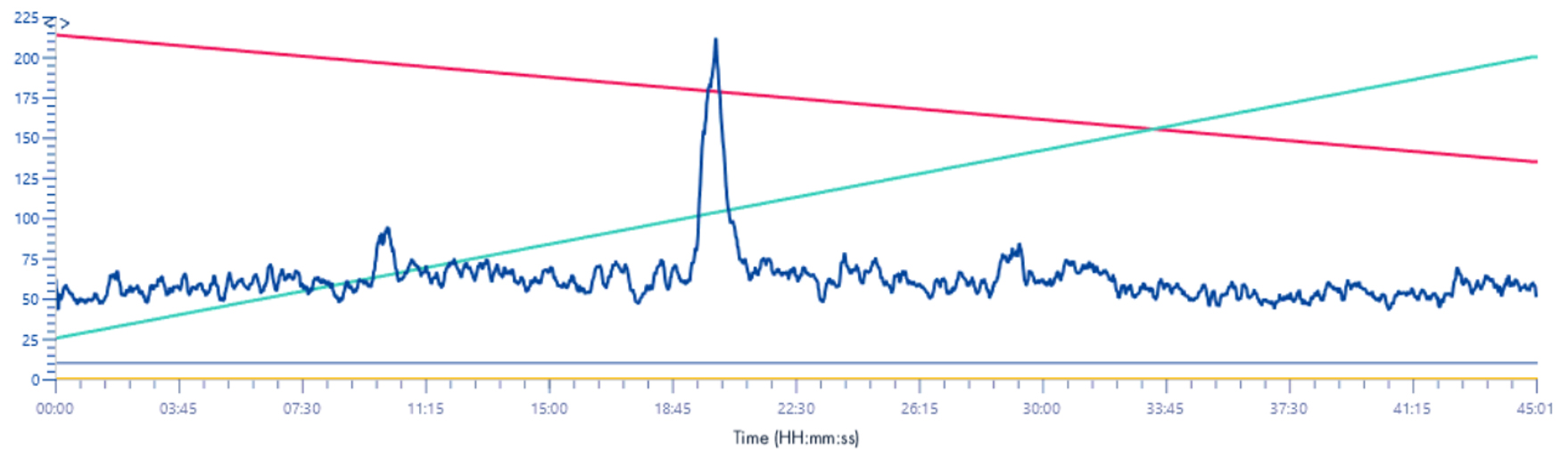
XIC signal 377-383m/z
Solvent make-up: Isopropanol
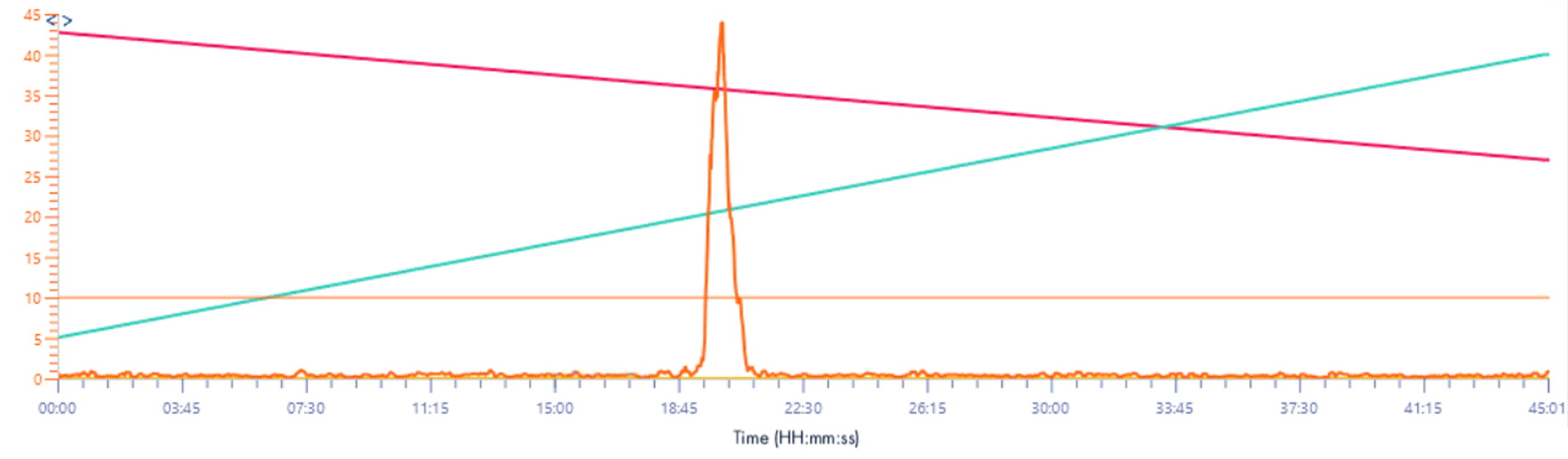
Solvent make-up: Isopropanol + 25% propanoic acid
Here the signal is not “lost” in the background noise, if you just use isopropanol as a make-up solvent.
When using propanoic acid we notice the same thing as before, the intensity is higher, about 10x as well.
XIC signal 571-577/z
Solvent make-up: Isopropanol
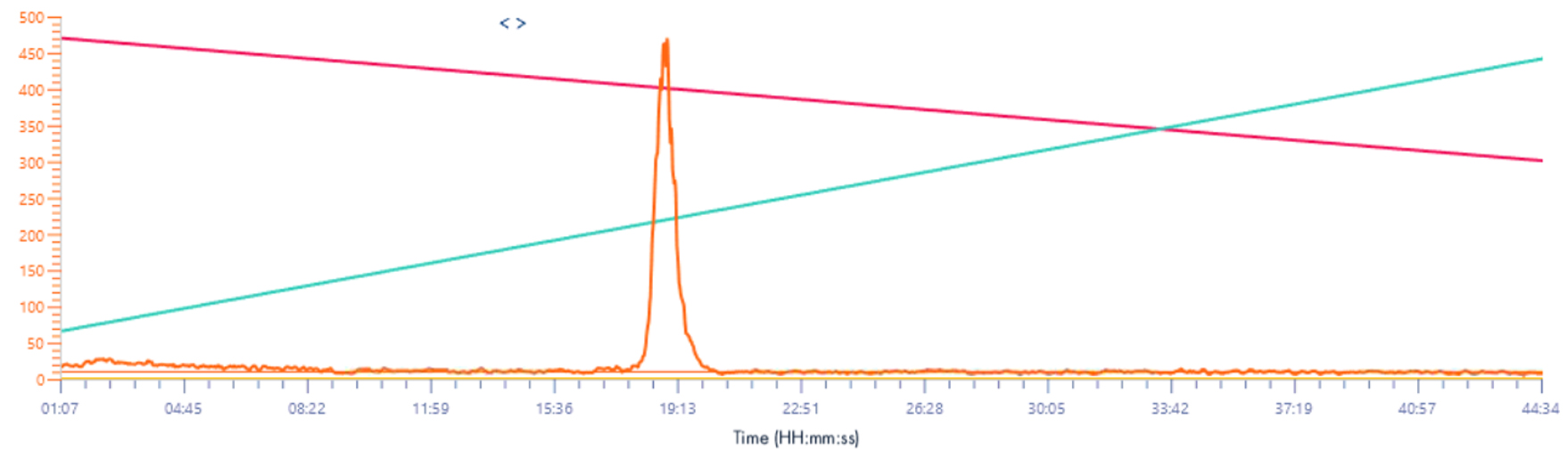
Solvent make-up: Isopropanol + 25% propanoic acid
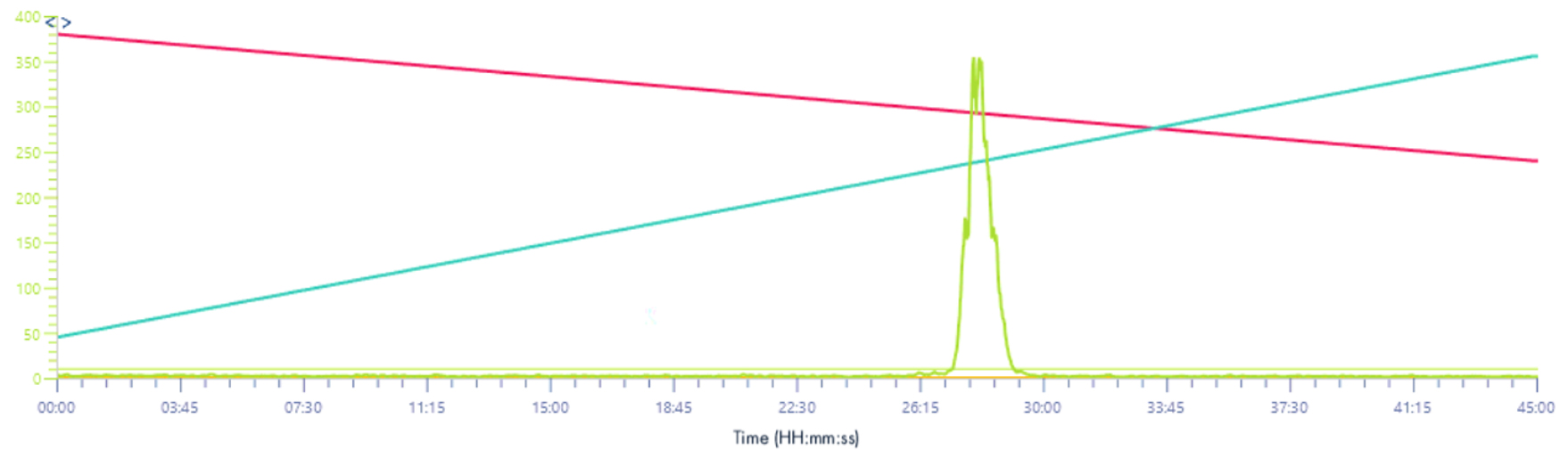
Same results than XIC 2 signal, the intensity is increase almost 10 times.
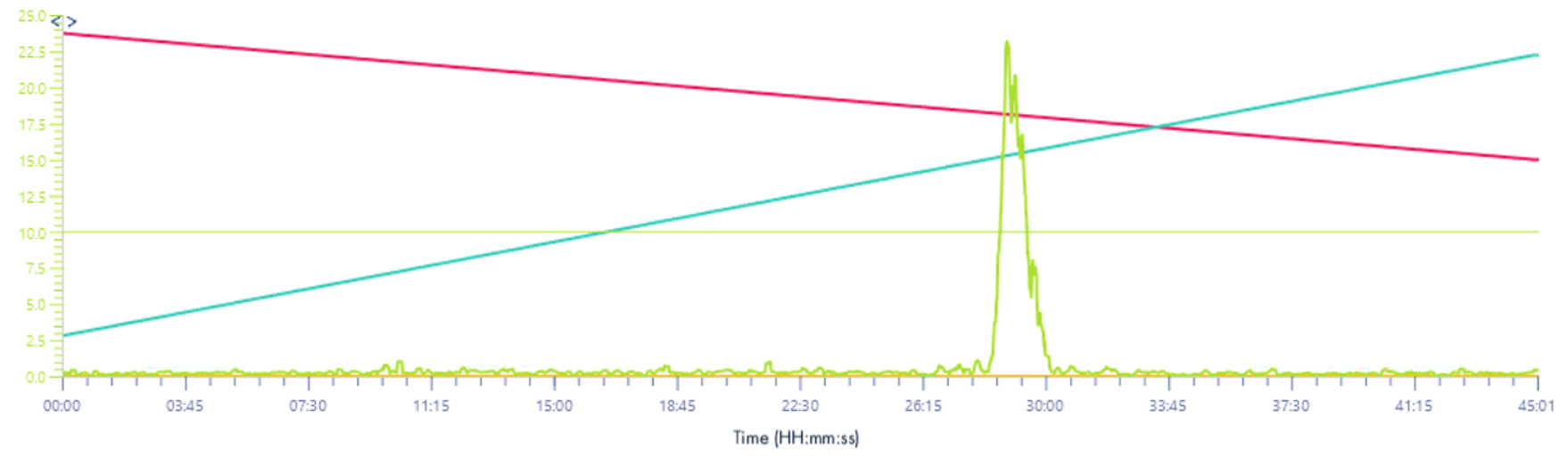
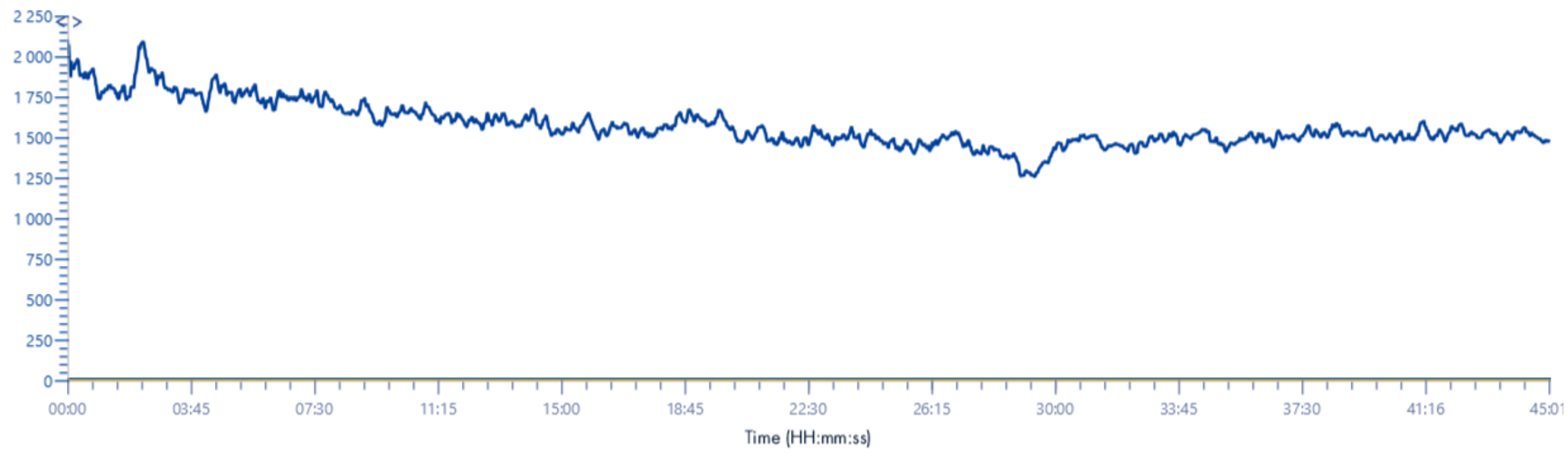
TIC 220-1200m/z
Solvent make-up: isopropanol
Solvent make-up: Isopropanol + 25% propanoic acid
Here, too, it can be seen that the use of propanoic acid allows a much clearer visualization of mass signals.
NB: For the TIC signal, we were obliged to increase the lower bound value of m/z range. Indeed, if this value is at 100m/z we no longer have a signal, as it is totally absorbed by the signals emitted between 100 and ~180m/z (see chromatogram below).
Conclusion
This trick can therefore be used in order not to have to redevelop new methods, and therefore saves a lot of time.
It is noted that we used propanoic acid, but what would be the result with other acids?
Formic acid, because of its too high volatility (higher than TFA), does not allow the visualization of signals.
Acetic, propanoic and butanoic acids give good results. Butanoic acid gives even the best results, however, because of the smell it gives off, its use is not recommended.
Carboxylic acids with larger carbon chains, due to their lack of volatility, give less good results.
Know more:
- Find our application notes available on www.flash-chromatographie.com/ressources/application-notes
- Discover Interchim® peptide purification systems : www.flash-chromatographie.com/peptide-oligonucleotide-purification-puriflash















