PEGylation: definition
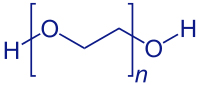 PEGylation refers to techniques that modify compounds or supports, adding polymeric chains of EthyleneGlycol (or PolyEthylene Oxide, or polyoxyethylene), as well as their applications. Introduced in the 1970’s to improve the drug bioavailability (life time in blood, reduction of immunogenicity), these techniques diversified, encompassing various applications in organic synthesis, biochemistry, analysis, purification, functionalization of surface in material sciences (bio-, nano-), cell culture…
PEGylation refers to techniques that modify compounds or supports, adding polymeric chains of EthyleneGlycol (or PolyEthylene Oxide, or polyoxyethylene), as well as their applications. Introduced in the 1970’s to improve the drug bioavailability (life time in blood, reduction of immunogenicity), these techniques diversified, encompassing various applications in organic synthesis, biochemistry, analysis, purification, functionalization of surface in material sciences (bio-, nano-), cell culture…
This article reminds some key advantages generated by PEGylation, developing physical features notably of PEO versus PEG compounds, and functional groups related to methods of PEGylation.
Principle and method of PEGylation
To pegylate a molecule or a support consists to graft (or conjugate) it with hydrophilic chains of PEG/PEO, to induce several physicochemical modifications at the molecular level, starting with hydrophilicity, but also the Molecular Weight, the steric volume or hindrance…
Corollary at macroscopic levels, one get physical, biochemical and biological modifications, that are taken to good account in industry (material), pharmaceutics (drugs, vaccines), medical (prothesis),…
The conjugation implies usually covalent links, however, it is sometimes done by adsorption, or trapping (gels).
Advantages of PEGylation
1/ Improved the molecules properties
Modifying a biomolecule by PEG or PEO a agent aims to improve its properties and behaviors at the molecular level, in the organism, or in a technique (analysis, purification, materials, probes). Typically, we can notice :
- Solubility and/or hydrosolubility are increased
- Steric and hydrodynamic changes (molecular interactions are improved in aqueous environment),
- Spacing effect (adjustable lengths)
- Non toxicity, weak immunogenicity, improved bioavailability…
- Compatibility with any chemistry of conjugation/labeling/functionalization
2/ Multiples characteristics, properties and applications :
Pegylation agents are polar, hence soluble in aqueous solutions that render them suitable for biological/biochemical applications. Superior concentrations can be performed during the conjugation step, allowing higher coupling ratios.
More interestingly, the hydrosolubility is carried by the spacing arm (and not by an additional polar group, such as sulfoNHS that is removed after coupling), hence the hydrophilicity is held by the formed conjugate :
- The hydrophilic PEG chains favorize the solubility of conjugates in aqueous buffers. This reduce aggregation that is sometimes observed using classical crosslinkers. This also avoids precipitations and increases the stability of the conjugate ;
- The hydrophilicity of a pegylated drug changes its distribution between compartments in cells and organism: it will generally be less sequestrated in fatty compartments and more bioavailable.
The motility is increased in a aqueous solution, modifying in general favorably the interaction of a drug or affine probes toward its target (enzyme, receptor on a biological membrane,…) - The molecular weight and size of a conjugate are increased, and can by modulated by the length of the PEO/PEG chain. Small PEG grafting can increase hydrosolubility with a limited growth in size. At the opposite, a drug pegylated by large PEG will not by filtrated by kidneys.
- The steric volume, notably hydrodynamic volume, is also increased. This allows to protect bioactive compounds against a proteolytic activity, change the properties of surface of a support and its interactions with solutes or other supports (anti aggregate effect for particles).
Very interesting macromolecular properties appear : absorption capacity and viscosity of hydrogels ; sequestration/release of active agents, amphipilicity of polymer alterning hydrophilic and hydrophobic motifs, CMC and aggregation number of micelles). - The pegylation agents can be branched. That allows to create original structures (ex. dendrons) in solution or immobilized. The pegylation then generates singular properties at the physical (through stericity), chemical and even biochemical (through multivalency) levels.
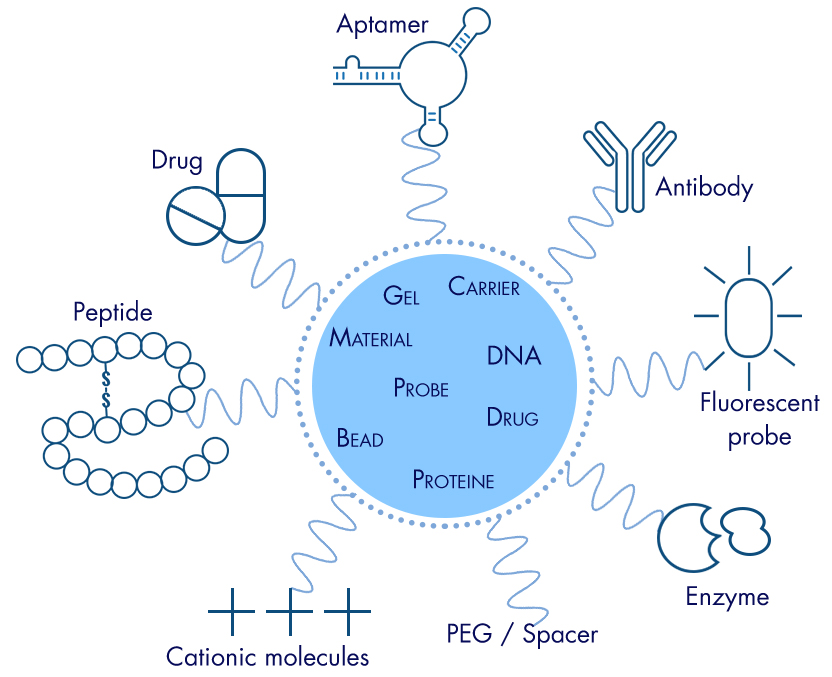
- Finally, PEGylation agents are available fonctionnalized by any groups used in classical chemistries (NHS/Amine, Maleimide/Sulfhydryl,…) and moderns (click chemistry). It is possible to graft labels (fluorophore, tag,…), affine groups, substrates, antigens, oligos or peptides, in a bioorthogonal manner (without interference with biological compounds / even in vivo). The conjugation can associate several functions, same or different, that combine or interfere in the PEG polymer or with other partners (AntibodyDrugConjugate, affinity + substrate, bispecific binding, FRET, molecular beacons,…).
The designs are infinite, assembling these functional groups linearly or not on PEG chains (or at the opposite : peptides multi-PEGylated), regularly (polymers) or not…
3/ Applications of PEGylation are numerous :
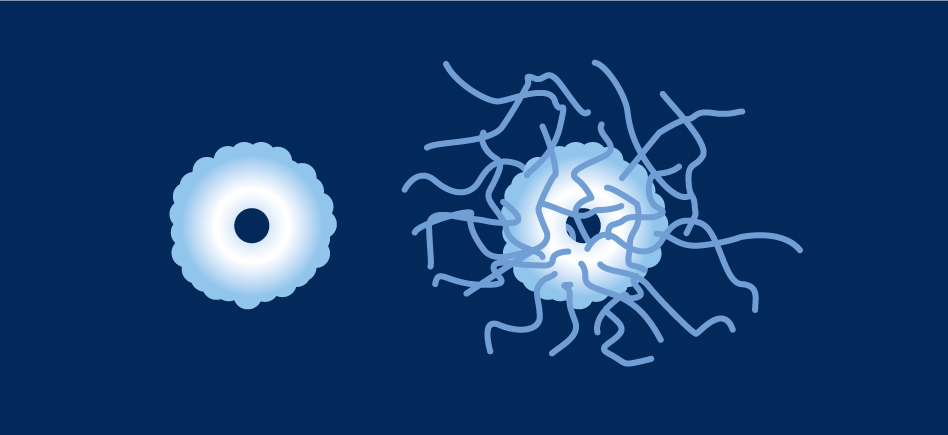
Comparison between uricase and PEG-uricase
PEG-uricase includes 40 polymers of 10kDa PEG.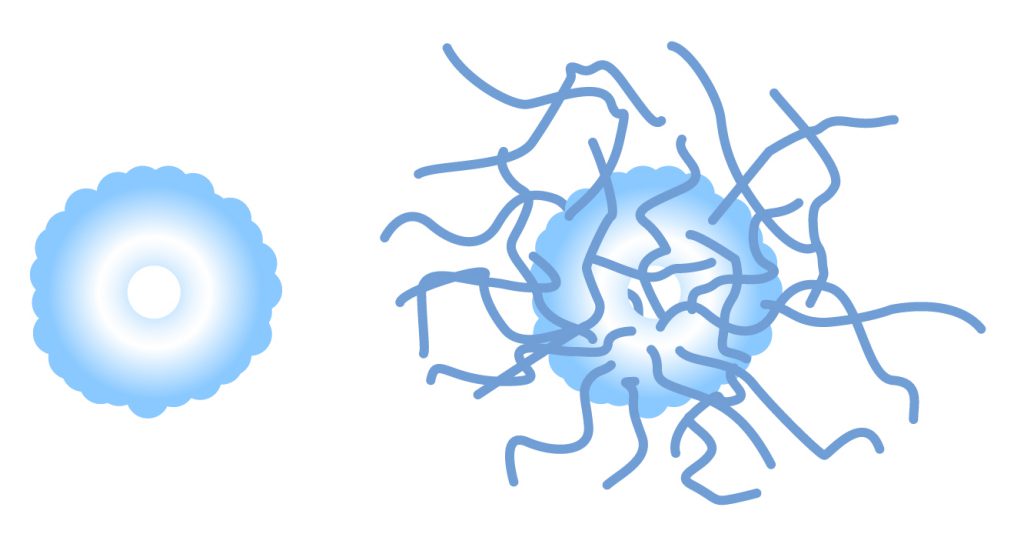
PEGylation :
- improves its solubility at physiological pH,
- increases serum half-life,
- reduces immunogenicity without compromising activity.
Pegylation PEO versus PEG :
 The PEGylation agents are obtained usually by polymerization and purification. They present then a certain dispersion of size/weight (MW), typically Gaussian-type with an index of dispersion (PDI) of 1.05 to 1.2, taht make +30 up to 100 different compounds.
The PEGylation agents are obtained usually by polymerization and purification. They present then a certain dispersion of size/weight (MW), typically Gaussian-type with an index of dispersion (PDI) of 1.05 to 1.2, taht make +30 up to 100 different compounds.
This dispersion, even weakest (~1%: for PEG said ‘monodisperse’, ‘homogeneous’, ~100 to 1250Da), generate a variability in conjugates, often adverse. It is rarely proposed PEG compounds that are perfectly defined at the molecular level (called ‘discrete’ or more exactly ‘single compound’).
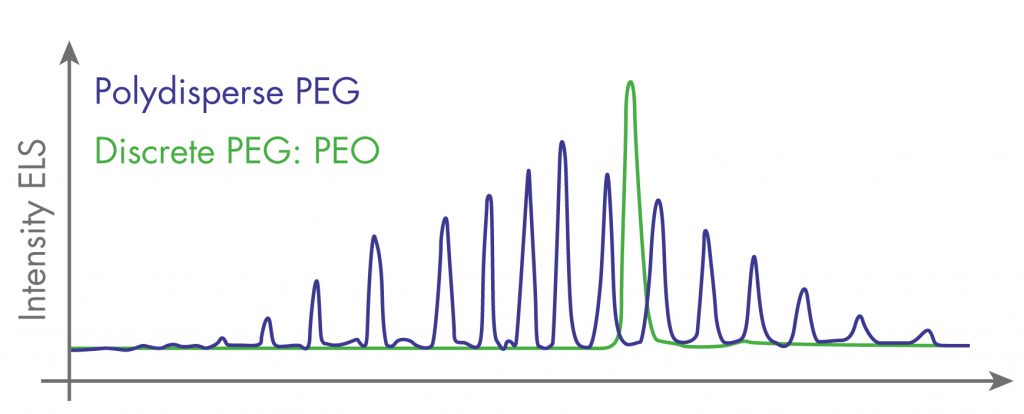
To avoid such ambiguous designations, Interchim qualifies :
- ‘PEO’ the former ‘single compounds’, made by synthesis (sizes up to 2KD, even 8KDa);
- ‘PEG’ the classical poly- and pauci-, even mono-disperses (from 0.2 to 300KD).
Chemistries of PEGylation :
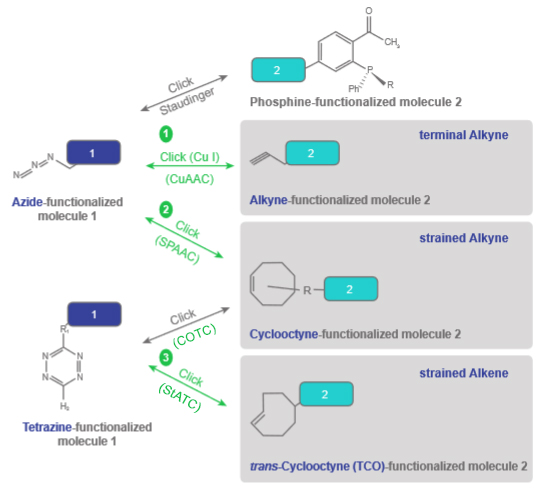 The covalent pegylation uses crosslinkers with PEG ou PEO spacers that are terminated by various functional groups, with classical coupling chemistries (NHS/MAL, EDC,…) or by click chemistries (Azide, Alkyne, DBCO, CHO, 4FB, HyNic, AminoOxy…).
The covalent pegylation uses crosslinkers with PEG ou PEO spacers that are terminated by various functional groups, with classical coupling chemistries (NHS/MAL, EDC,…) or by click chemistries (Azide, Alkyne, DBCO, CHO, 4FB, HyNic, AminoOxy…).
The latter chemistries, said bioorthogonal (not interfering with biological compounds), present a specificity and an orientation of the chemical coupling, while versatile and controllable.