The Lucent360™ LED modules and vial holders/reactors are interchangeable allowing the user to design the perfect experiment to fit their needs. With variable wavelength, light intensities vial arrangements and temperature control, the flexibility available to design a custom optimization are seemingly endless. The Lucent360™ allows you to quickly investigate the reactions parameters necessary to scale up a reaction at small scale with production using the same instrument.
A demonstration of the flexibility afforded by the Lucent360™ is shown by the following reaction light intensity and catalyst screen for the iridium catalyzed C-C bond formation (see Figure 1).
Figure 1 :

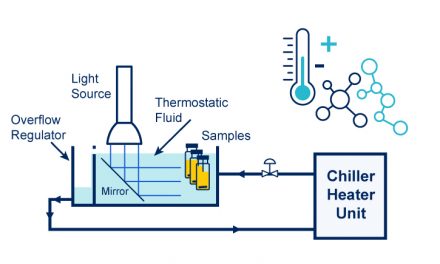
Using the Lucent 360™ multi-light screener (HCK1021-01-010) capable of screening 16 reactions at 4 different wavelengths and light intensities, the following reaction screen was performed at 450 nm. Four iridium catalyst concentrations (1 mol %, 0.5 mol %, 0.1 mol %, 0.01 mol %.) were performed with four different light intensities on the Lucent360™. Based on the actinometry experiments performed in 2 ml reactions, each setting corresponds to running the reactions at 0.25 W, 0.54 W, 1.05 W and 1.2 W effective power. These effective powers correspond to photon flux ranging from 9.5×10-7 to 4.5×10-6 Einstein/s (moles photons/second).
| Effective Power/Reaction (W) |
(+/-) | Photon Flux (Einstein/s) | |
| Quadrant 1 | 1.21 | 8.4% | 4.54E-06 |
| Quadrant 2 | 1.05 | 8.3% | 3.94E-06 |
| Quadrant 3 | 0.54 | 6.0% | 2.03E-06 |
| Quadrant 4 | 0.25 | 4.1% | 9.50E-07 |

| Effective Power/ Reaction (W) |
(+/-) | Photon Flux (Einstein/s) | |
| Quadrant 1 | 1.21 | 8.4% | 4.54E-06 |
| Quadrant 2 | 1.05 | 8.3% | 3.94E-06 |
| Quadrant 3 | 0.54 | 6.0% | 2.03E-06 |
| Quadrant 4 | 0.25 | 4.1% | 9.50E-07 |
The multi-light intensity/catalyst screen was performed according to the following protocol (Table 1):
To each 4 ml vial equipped with a Teflon septa and stir bar was added a solution containing 1.1 mg Ni-glyme (5 mol %, 5 µmol) and 1.3 mg dtbbpy (5 mol %, 5 µmol) in methanol and the solvent was removed under reduced pressure (16 vials). To the resulting vial was weighed 48.9 mg Cs2CO3 (1.5 equiv., 150 µmol under inert atmosphere and capped. To this vial was added a 1 ml sparged solution in DMSO containing 19.9 mg bromoacetophenone (100 µmol), 35.6 mg Boc-Val-OH (1.5 equiv., 150 µmol) and 7.7 mg biphenyl (0.5 equiv., 50 µmol) as an internal standard. Ir(dF-CF3-ppy)2(dtbbpy) was added as a solution in DMSO to afford 0.01, 0.1, 0.5 or 1 mol % catalyst. Additional DMSO was added to each reaction to bring total reaction volume to 2.0 mL DMSO. Reaction was performed at 30 °C in the Lucent360™ with 450 nm LED. Analytical samples were taken from each vial at 5, 15, 30 and 60 minutes for analysis by LC-MS (10 µL dilution to 1 mL in DMSO).
Table 1 : Experimental Details (for standard conditions)
| Reagent | Equivalent | Amount (µmol) | 0.05 | M |
| bromoacetophenone | 1 | 100 | 19.9 | mg |
| boc-Val-OH | 1.5 | 150 | 32.6 | mg |
| Ir(dF-CF3-ppy)2(dtbbpy) | 0.01 | 1 | 1.1 | mg |
| Ni-glyme | 0.05 | 5 | 1.1 | mg |
| dtbbpyp | 0.05 | 5 | 1.3 | mg |
| Cs2CO3 | 1.5 | 150 | 48.9 | mg |
| Biphenyl | 0.5 | 50 | 7.7 | mg |
| solvent | DMSO | 0.05M | 0.002 | L |
Monitoring the reaction screen for 1 hr demonstrated clear trends in the product formation in relation to the catalyst concentration and light intensity (Table 2). Each reaction with 1 mol % Ir catalyst resulted in similar conversions (62-65%) at 60 minutes independent of the power setting. With 0.5 mol % catalyst 0.54 W or higher was sufficient to achieve a similar conversion. Lower catalyst loadings were detrimental to the conversion regardless of power setting.
Tableau 2 : Conversion at 60 minutes
| Power (%) | ||||
| Ir cat (mol%) | 0.25W | 0.54W | 1.05W | 1.21W |
| 1 | 62.0 | 63.5 | 65.6 | 65.2 |
| 0.5 | 47.8 | 65.1 | 67.7 | 65.8 |
| 0.1 | 15.7 | 33.7 | 45.2 | 47.7 |
| 0.01 | 1.0 | 0.2 | 2.6 | 2.6 |
While final conversion is similar for the seven conditions highlighted in green in Table 2, significant differences were observed in the initial rates and time to reaction completion (Figure 3). Both reactions at 1.0 W and 1.2 W are completed by 30 minutes with minimal additional product formation while the two lower power settings take the full 60 minutes for completion.
Figure 3: Plot of time course for 1 mol % at 0,25W, 0,54W, 1,05W et 1,21W

Based on the results, the reaction can be performed with 0.5 mol % photocatalyst and 0.54W to achieve similar conversions to higher catalyst loading and light intensity (Figure 4) albeit with a slower reaction.
Figure 4: Plot of time course for 0.54 W setting with 4 catalyst concentrations

Monitoring the initial rate of the reaction at 5 minutes, we can begin to see clearly the significant effect of the light intensity on the reaction. Increasing the initial rate of the reaction is a key parameter in determining the appropriate conditions to scale up a photochemical reaction. What does this all mean for this C-C bond formation reaction? We’re not sure yet, but we know that without the easy setup of the Lucent360™ and the ability to monitor multiple light intensities at the same time, it would have taken us significantly longer to get all this data. Maybe for our next experiment using this reaction, we could look at 4 different concentrations of the nickel co-catalyst at each light intensity in comparison to the 0.5 mol % iridium catalyst, or screen or add additional wavelengths (365 nm, 380 nm, 405 nm or change the concentration of the substrates. Whatever we decide, we know that it will be easy to perform with the Lucent360™.
Figure 5 :

Know more:
- Contact us: interfine@interchim.fr
- Acess directly to the Lucent360™ from Hepatochem on our website.
- Follow our news on LinkedIn