| Click chemistry involves specific, convenient and biocompatible reactions of 2 chemical groups (Click partners) that are not native of biomolecules and, so, will not interfere with the chemical groups of samples, nor with the biochemical reactions in living systems (what is called ‘bioorthogonality’)*. The Click term is now coiled notably for the controlled conjugation of molecules, and especially of biomolecules even in complex mixtures such as biological samples. This makes Click chemistry widely applied, in-vitro and in-vivo, for conjugation of natural compound or xenobiotics, together, or with a label or a support, as a all-purpose method to make probes for general or specific detections, or also for purification applications. |
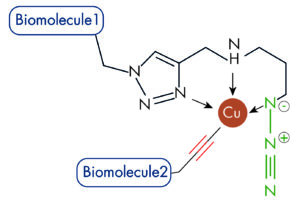
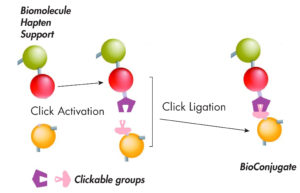 Figure 1: Principle of bioconjugation by Click chemistry |
*rem: Click reactions are operated in some particular modalities to interfere with biological processes, and so are used as pinpoint tools to study them.
Click chemistry confers remarkable benefits:
- Simple to perform – Modular – Scalable
- Very selective and Wide in scope – Work in aqueous, pH insensitive – Bioorthogonal
- High yielding, thanks to reaction high thermodynamic force: > 84kJ/mol – Easier purification
- Stereospecific, can be chemo-, regio-, diastereo- and enantio-selective
- Yields physiologically stable products
- Adhere to the several green chemistry principles, including non toxic solvents and ‘atom economy’.
This article outlines:
- An overview of the main techniques in Click chemistry
- Insights about innovative Alkyne/Azide CycloAdditions (AAC):
- New catalyzers for CuAAC,
- New alkyne derivates – CPAAC
- New azide derivates – SPAAC
- Other highlights: cleavable spacers, clickage supports
Main Click chemistry techniques for bioconjugations
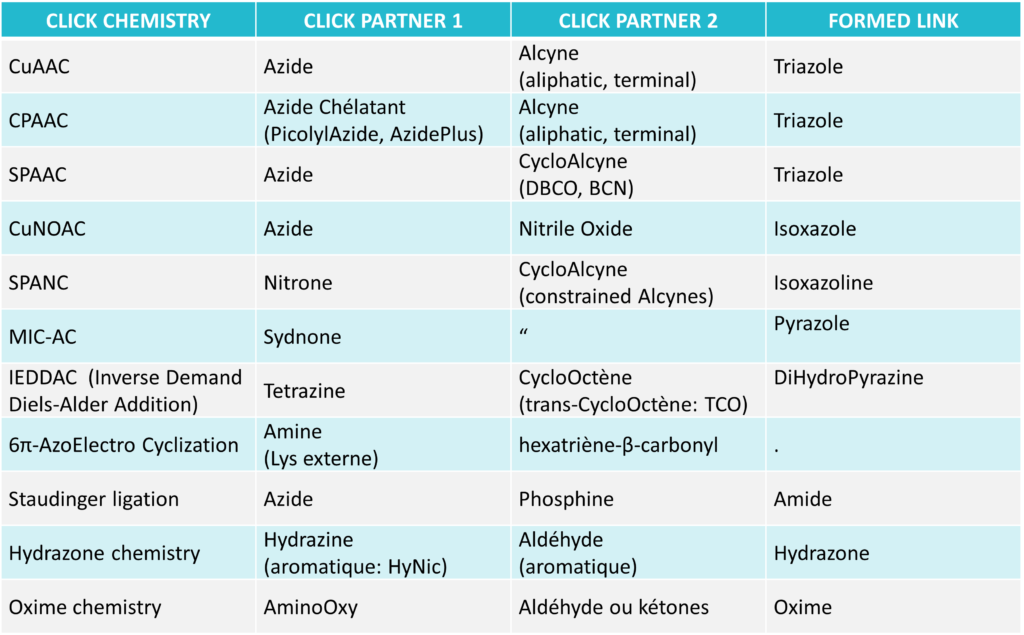
The following table distinguishes several types of Click-type chemistries for bioconjugation:
Rem: other kinds of Click are not included here: other azide-alkyne cycloadditions such as Huisgens AAC, RuAAC, IrAAC; other 1.3 1,.3-Dipolar CycloAdditions; MesoIonic compounds (sydnone imines, münchnones, mesoionic carbenes); diels-Alder Cycloadditions; TAD Click (triazolinedione) and Trans-Click Reactions; Thiol-X reactions (Thiol-ene and Thiol-yne); SuFEx reactions
The following will deal mainly about AAC click chemistry (Azide-Alkyne Cycloadditions).
Advanced click chemistry for bioconjugations – Insights
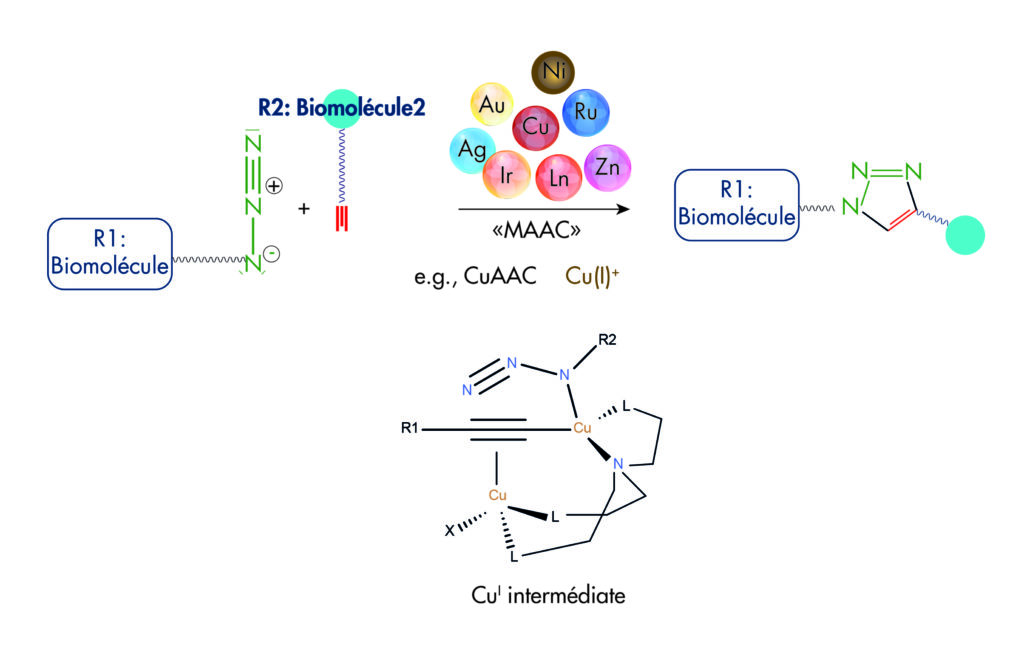
Introduction. Standard click reactions CuAAC
Click chemistry was introduced by Sharpless in 2001 to describe reactions that have high yields, are wide in scope, and create products easy to recover by, and preferably without chromatography. This Azide / Alkyne cycloaddition, originating to Michael and Huisgen reaction (1893), is known as ‘CuAAC’ (Copper ion catalyzed AAC) and now related to many more reactions catalyzed by several metals (Metal-catalyzed Azide-Alkyne Additions : MAAC).
In the emerging field of click chemistry applied to bioconjugation, many efforts led to address several limitations. Improvements came through new catalyzers, then new alkyne derivates and new azide derivates able to react without catalyzer, or quicker, and also through added functionality such as spacer cleavability or traceabilty.
See Click Chemistry reagents:
• standard Alkyne reagents for CuAAC (and CPAAC)
list of Alkyne Crosslinkers | Alkyne Biotins | Alkyne Fluorescent agents
from Uptima | FluoProbes | All
• standard Azide reagents for CuAAC (and Staudinger ligation)
list of Azide Crosslinkers | Azide Biotins | Azide Fluorescent agents
from Uptima | FluoProbes | All Azides
1. Improved click reactions by superior Catalyzers and Buffers for CuAAC
Because the standard Click chemistry (CuAAC) use catalysis by copper as degree of oxidation Cu(I), that is not available as convenient salts and well stable, Click reactions were obtained using Cu(0) oxidized to Cu(I) by ammonium salts or triethylamine chlorhydrate. But it became more convenient to generate Cu(I) from Cu(II) salts, by reducers like Cu(0) (copper metal) and more popularly by Ascorbic acid. Superior additives to improve the click reaction were then proposed, mainly triazole chelating agents such as TBTA , BTTAA . Lately the THPTA reagent provides benefits to Click reaction similar to BTTAA, plus water-solubility, that simplifies click chemistry by allowing the entire reaction to be run in water. A DNA enzyme was also proposed to harness as low as sub-micromolar Cu+ catalyzer.
Uptima Click accessory reagents :
- Cu2+/TBTA complex #FY2780; Ready-to-use catalyzer for CuAAC.
- THPTA #APIFZA ; Tris((1-Hydroxy-Propyl-1H-1,2,3-Triazol-4-yl)Methyl)Amine; CAS: 760952-88-3 ; MW: 434.50.
BTTAA #APIFU2 ; Tris(3-hydroxypropyltriazolylmethyl)amine; CAS: 1334179-85-9 ; MW: 430.52. - Copper(II)-Sulphate (CuSO4), #1H3691; CAS: 7758-98-7; MW: 159.6.
- Sodium Ascorbate (Na-Ascorbate), #APIG40 ; CAS: 134-03-2; MW: 198.1.
 |
 |
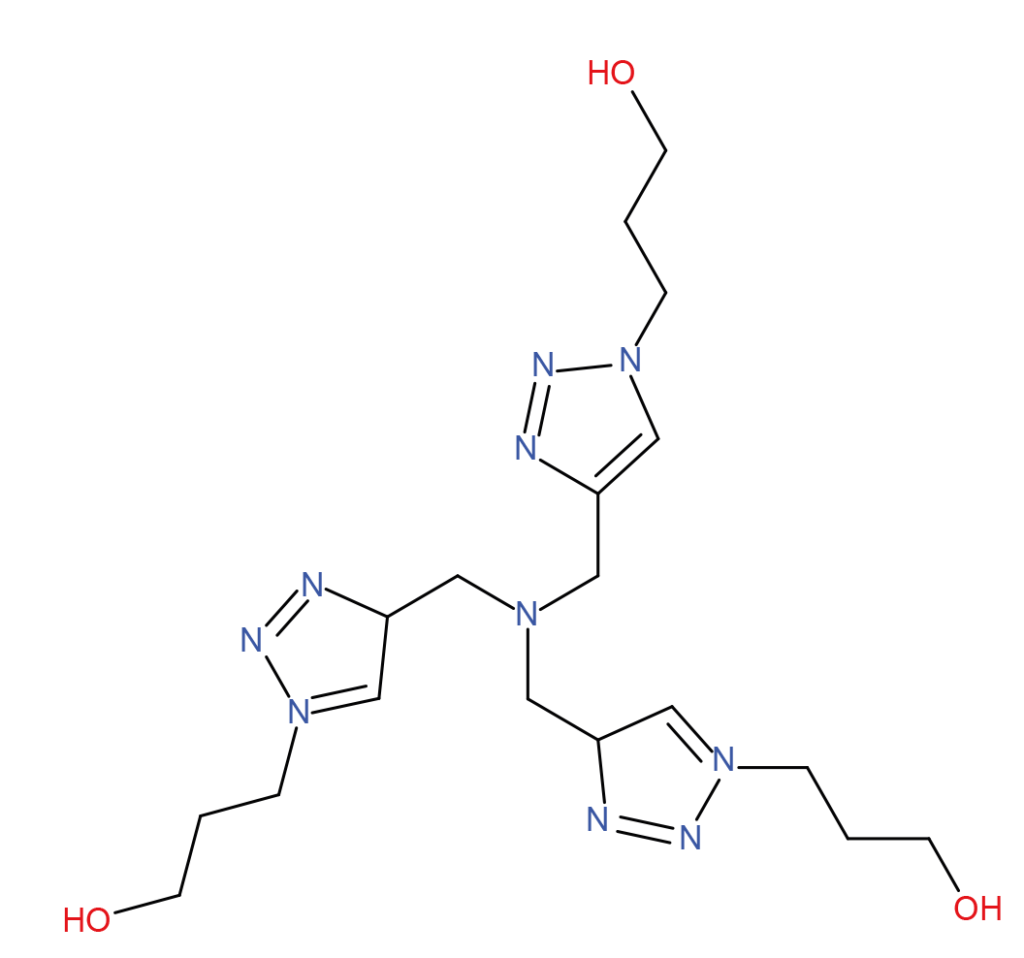 |
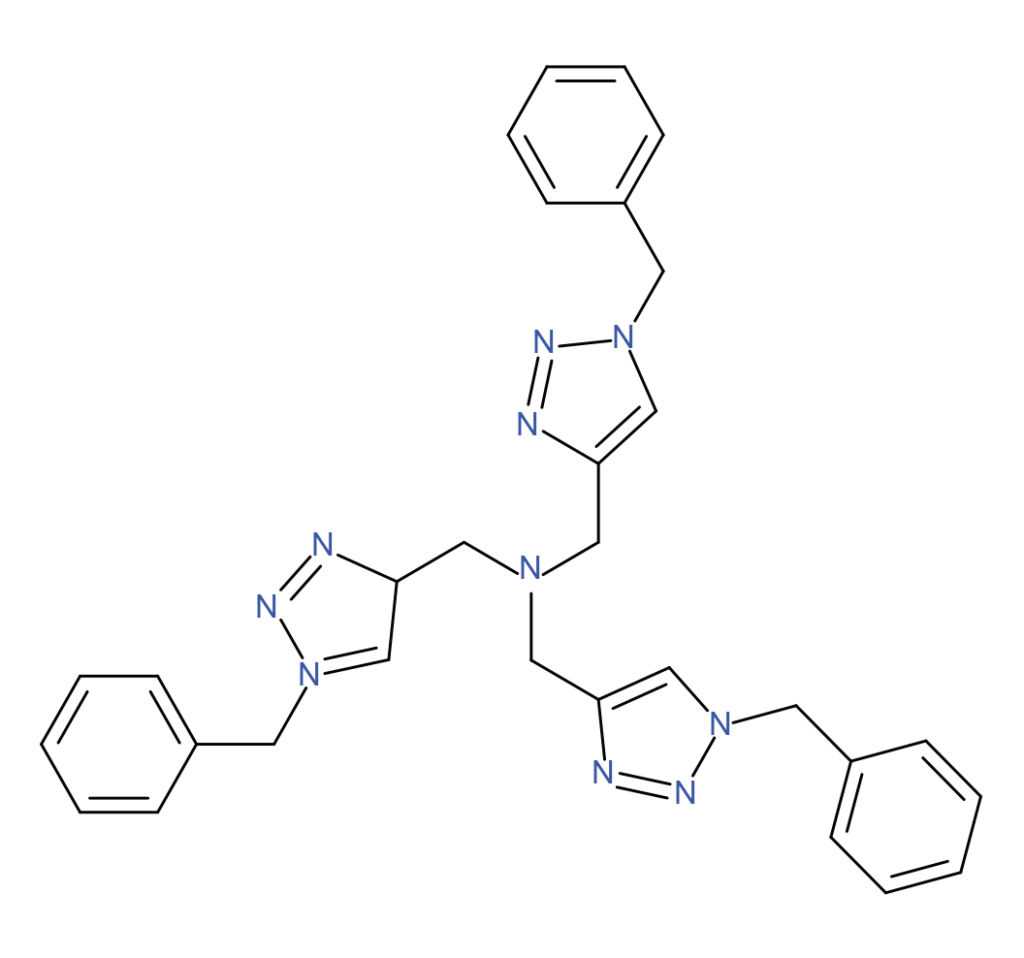
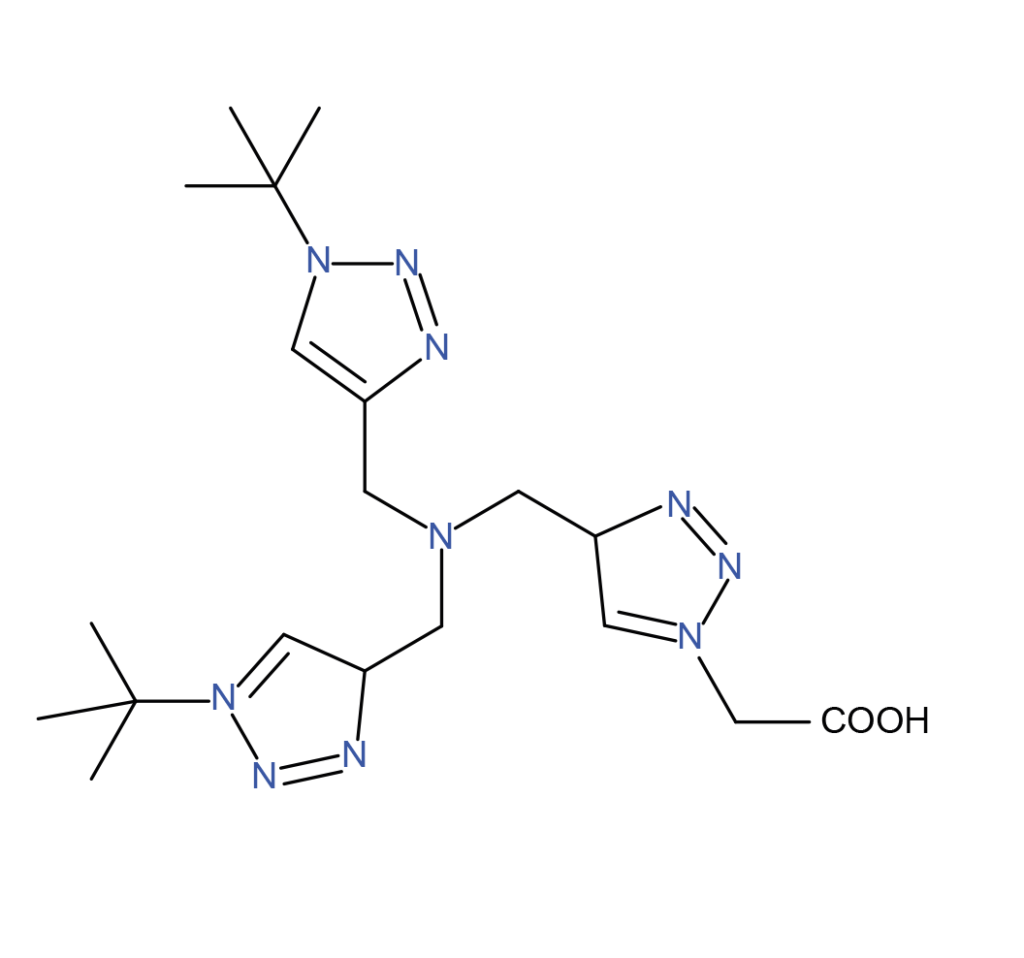
| TBTA | THBTA | BTTAA |
Alternatively to chemical catalyzers, the photo-induction of Click reaction has been well developed, opening a new field of applications (not discussed here – see further article).
2. Improved click reactions due to Advanced Azide derivates : CPAAC
Several new Azides were proposed, because Cu(I)(II) ions were deleterious in biological applications (toxicity), but also to speed up the CuAAC click reaction:
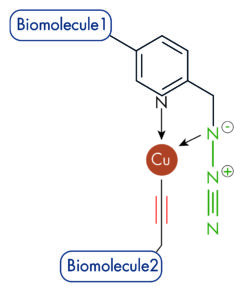
| Picolyl-Azides: The picolyl-azide features a copper-chelating moiety that raises the effective Cu+ concentration at the Click-reaction site. Nevertheless, the copper-chelating motif of picolyl azide molecules require the presence of a copper chelator (e.g. THPTA) to achieve significant improvement in the kinetics of the CuAAC reaction. |
|
| AzidePlus: The Azide plus is the next generation of copper+-chelating functionalized Azide. It is the fasted reported structure to date, and does even no more require the presence of copper-chelating ligands. |
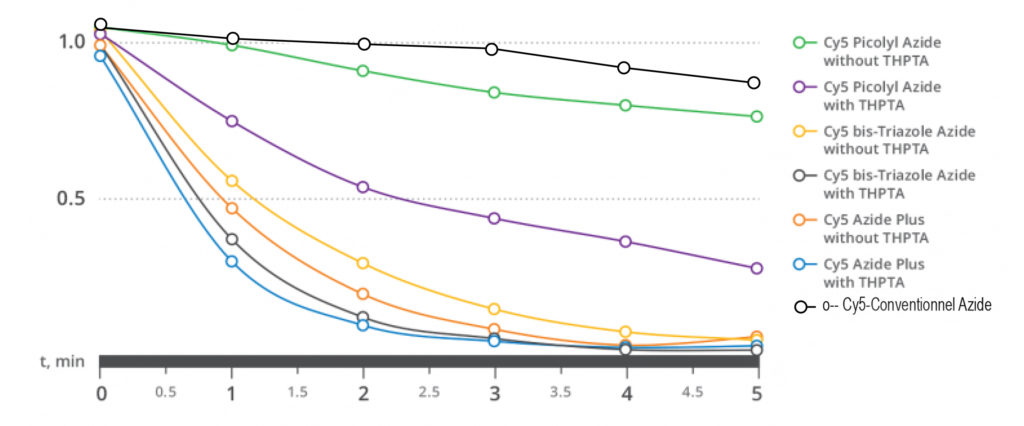
Figure 5 : kinetic comparison of AAC click reaction with 3 different spacing arms in a conjuguate fluorophore (Cy5) – azide. The click reaction on an agarose-alkyne resin makes the fluorescence disappear from solution, much quicker with AzidePlus than with spacers Picolyl or Triazole, to an extend where it is no more significantly improved by the added THPTA ligand.
Click chemistry using PicolylAzide and AzidePlus is referred as CPAAC (Chelation Promoted Azide/Alkyne Conjugation), and yields superior bioconjugations over standard Click reaction (CuAAC).
See Advanced Azide reagents for CPAAC:
• List of Picolyl Azides reagents:
FluoProbesDyes Picolyl (all/more); CFdyes ; TAMRA PicolylAzide; FAM PicolylAzide; Cy3/5/5.5/7 PicolylAzide
• List of AzidePlus reagents
List of AFDyes AzidePlus and AFDyes PicolylAzide
(more AFDyes: functionalized by –Alkyne, –DBCO, –Tetrazine, –TCO, –NHS, –Cadaverine, –Biotin)
• Alkyne reagents: see above
3. Improved click reactions due to Advanced Alkyne derivates – SPAAC
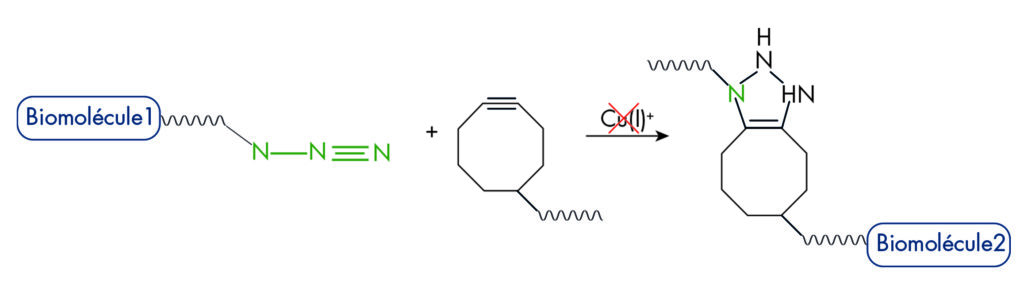
An other approach to increase the reactivity of alkyne and avoid the use of toxic copper catalysts was first introduced by Bertozzi for bioconjugation, using constrained alkynes.
Several kinds of strained alkynes were proposed, like nonynes (BCN), and more interestingly cyclooctynes moiety (DBCO, DIBO). These novel reactions do not require a cytotoxic Cu(II) catalyst, “Copper-free”, and are very fast at room temperature. They are called Strain-promoted alkyne-azide cycloaddition (SPAAC). Cyclooctynes are thermostable with very narrow and specific reactivity toward azides, resulting in almost quantitative yields of stable triazoles.
See SPACC reagents:
• DBCO reagents
list of DBCO Crosslinkers | DBCO Biotins |DBCO Fluorescent agents
from Uptima | FluoProbes | All
• BCN reagents
list of Crosslinker | Biotin | Fluorophores (CF dyes)
from Uptima | Biotium |
• Azide reagents: see above
4. Advanced click reactions with cleavability (photo-, or chemi-cleavable)
Several clickable reagents are available with a spacer: they make conjugates that first interact with ligands of interest, then can be cleaved in controlled way. The ligand(s) complexed to the conjugate is(are) so released and can be identified by mass spectrometry, ELISA, dot blot or Western blot analysis. Such Click cleavable-reagents allow also efficient recovery of the bound ligand(s) (pull-down assays).
Photo-cleavable click reagents: PC
The spacer arm containing a PhotoCleavable (PC) moiety can be efficiently cleaved upon UV exposure: typically >90% is clived in 5-25 minutes using an inexpensive, near-UV, low intensity lamp (e.g. 365 nm lamp at 1-5 mW/cm²).
PC DBCO : DBCO- PC-Biotin #AXBKW1, DBCO- PC-PEO3-Biotin #XEU461 – TechSheet #DQP701.
PC Azide : Biotin-PC-Azide #AXBKU1
Chemo-cleavable click reagents: SS, Diazo, Dde
The Disulfide (-S-S-) bridge can by cleaved by reduction with thiols (bME, DTT, TCEP,…).
Disulfide Biotin agents (& -DBCO #DQP701, -Azide #AXBJQ, -Alkyne #BX1LR)
The Diazo moiety can be cleaved under mild conditions with 50 mM sodium dithionite solution (Na2S2O4).
Diazo Biotins(& -Alkyne #MRT891, -Azide #MRT881; -DBCO #MRT901)
The Dde moiety can be cleaved by hydrazine under mild conditions (2% aqueous hydrazine).
Dde Biotins(& -Alkyne #XEU42A, -Azide #XEU421; -picolyl Azide #AXBLM1; -DBCO #XEU41)
Dde Fluorescent agents(& -TAMRA-Alkyne #BX1LY; TAMRA-Azide #BX1LZ; DPADA #30416A)
5. Clickable immobilizing supports
Click-functionalized agarose is a convenient tool to prepare custom affinity supports with the hapten or protein of your choice being immobilized, for the purpose of purifications or pull-down assays.
Typical matrix is 6 % cross-linked agarose as spherical beads of 50-150 μm size, with a degree of substitution of 5 – 20 μmol clickable groups/ml resin. It is ideal for purification of biomolecules, that is biocompatible and do not adsorb them unspecifically like silica, polystyrene or other polymers can present, thanks its natural hydrophilicity.
See Clickable Agaroses: -Azide € AXBI7;-PC-Azide #AXBKQ0; Alkyne #AXBHZ & MRV260, Dde Alkyne #AXBJK ; PC Alkyne #8K6942; DBCO #MRV270 & AXBJA; 4FB #IV2510
Search other functionalized Agaroses.
6. Click reactions alternative to AAC
Other groups than Azides & Alkynes used in AAC allow for interesting click reactions to bioconjuguate 2 compounds:
- Nitrile Oxides: Cycloaddition of Alkynes with NitrileOxide. It is less investigated, and needs Cu(I) catalysis (CuNOAC) unless using strained alkynes: the cycloaddition of 1,3-nitrone on the cyclooctyne DBCO (SPANC) yields a triazole link that is less stable than the AAC reaction, but is still very useful in some labeling applications where fastest reaction kinetics are critical. Same with other mesoionic compounds such as FluoroSydnones (K up to 104M-1s-1, surpassing those documented in the literature with cycloalkynes).
- Tetrazines: helpful click-like reactions are obtained with constrained alkynes, including the Inverse Demand Diels-Alder reaction (IEDDA). Remarkable groups include MethyTetrazine, PCC…
- Phosphines: helpful click-like reactions occur with Azides (Staudinger ligation)
- Hydrazines: helpful click-like reactions are obtained between aromatic hydrazines and aromatic aldehydes (Hydrazone Chemistry).
- Oximes & Aldehydes/Ketones: despite not completely abiotic, this type of reaction is interesting for specific applications (metabolic labeling to study intracellular processes, and glycan biosynthesis), and also because aldehydes can be introduced into proteins by several methods.
These will be the subject of next articles.
Know More:
- Search crosslinkers online
- Contact us for any technical, applicative or commercial support:
- Phone: +33 4 70 03 73 06
- Email : consumables.eu@advion-interchim.com