Detectors distinguish compounds from the mobile phase according to their physical properties. The collected fractions are cutted based on the dectector signal. Collect can be done relatively to a threshold and/or a slope value. Detection sensitivity is different from a detector to another, and is linked to compounds concentration or not. To maximize the dectection potential it is recommanded to couple different devices ie. UV+ELSD, UV+ELSD+MS,…
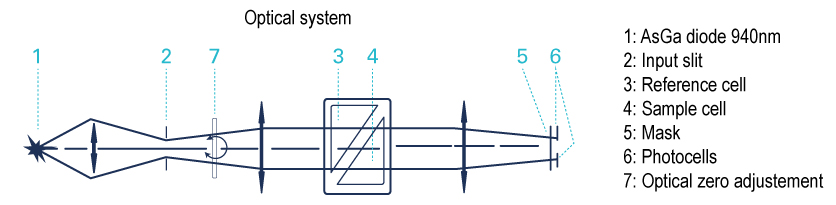
1. UV Visible Detector – Diode Array (DAD) Technology
 When subjected to a light radiation, certain functional groups may be the seat of electronic excitation corresponding to an energy absorption at a specific wavelength. The signal collected correspond to a light absorption.
When subjected to a light radiation, certain functional groups may be the seat of electronic excitation corresponding to an energy absorption at a specific wavelength. The signal collected correspond to a light absorption.
The UV detectors are not universal detectors. To be detected a compounds must have a chromophore in its molecular structure (i.e substances with aromatic ring, with at least 2 conjugated double bonds, with a double bond adjacent to an atom with ion electron pairs, with carbonyl groups, or containing bromine, iodine, or sulfur).
 Different UV detectors are commonly used:
Different UV detectors are commonly used:
- Detector with a fixed wavelength, managed by a specific lamp. In this case, compounds response must be verified.
- Detector with variable wavelength allowing to choose between several wavelengths. This leads to a maximum of sensitivity.
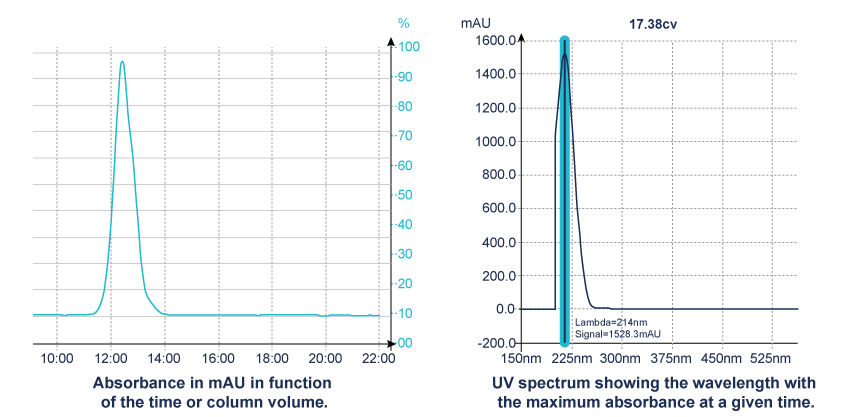
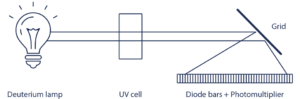
- Diode Array Detector (DAD), uses hundreds of diodes to scan a range of wavelengths and gives a 3D representation (time, absorbance, wavelength) of the signal. This detector allows the acquisition of the UV spectrum that give indication of the purity of each detected compound.
All wavelength detection:
When the maximum absorbance of molecule is unknown, the scan function of the detector is the right solution.
This scan signal corresponds to an average absorbance based on wavelengths within the range selected.
| Solvent | UV (nm) Cutoff @1AU | Solvent | UV (nm) Cutoff @1AU | |
| Acetone | 330 | Cyclohexane | 200 | |
| Acetonitrile | 190 | 1,2-Dichloroethane | 235 | |
| Dimethylformalmide | 268 | Dichloromethane | 235 | |
| Dimethyl sulfoxyde | 268 | Ethyl Acetate | 260 | |
| 1,4-Dioxane | 215 | Diethyl ether | 220 | |
| Ethanol | 210 | Heptane | 200 | |
| Isopropanol | 120 | Hexane | 200 | |
| Methanol | 205 | Iso-octane | 215 | |
| Tetrahydrofuran | 215 | Methyl tert-butyl ether | 210 | |
| Water | 200 | Butanone | 329 | |
| Benzene | 280 | Pentane | 200 | |
| n-Butanol | 254 | Toluene | 285 | |
| Carbon Tetrachloride | 263 | Xylene | 290 | |
| Chloroform | 245 |
Limits of detection:
he mobile phase can also interact in the detection and can absorb at a specific wavelength => solvent cut off.
Every compounds have their own molecular extinction coefficient.
Due to this property, the apparent equivalent absorbance of 2 compounds should lead to 2 different concentrations.
According to the Beer-lambert law, absorbance of each molecule will be linked to its own concentration.
A = ƹ C I
C = concentration in mol/L
l = cell length in cm
ƹ = molecular extinction coefficient (L.mol-1.cm-1)
The absorbance is improved when the cell path is increased.
2. Evaporative Light Scattering Detector (ELSD)
 Principle:
Principle:
ELSD detection is a three steps process:
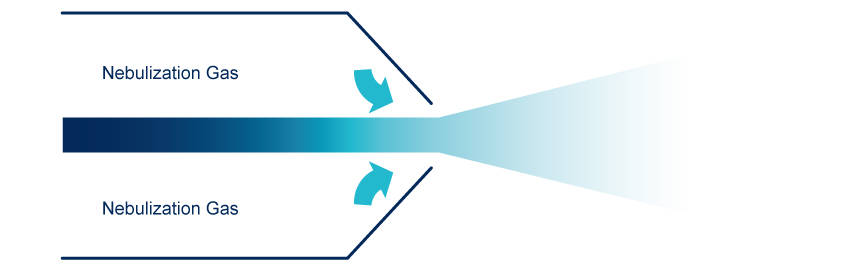
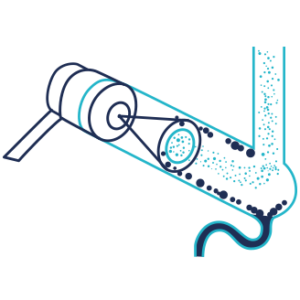 Nebulization
Nebulization
The nebulization is done, in a nebulization chamber, with a venturi nebulizer that generate droplets of mobile phase containing the compound of interest, the largest droplets are eliminated. Compressed dry air or Nitrogen are used as nebulization gas.
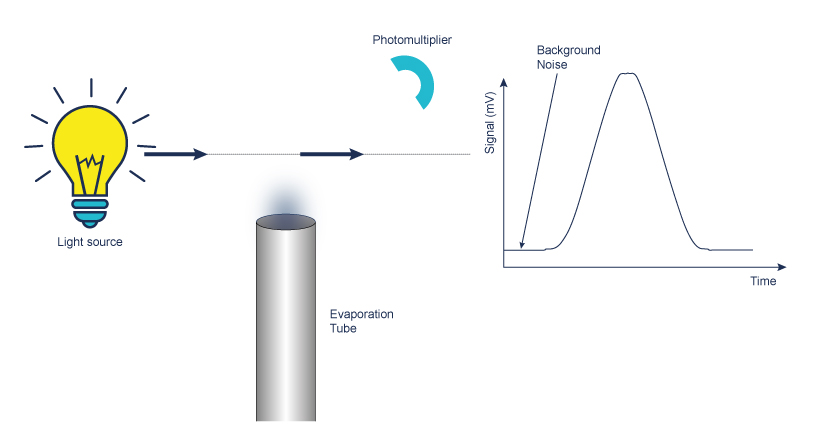
 Evaporation
Evaporation
The evaporation is set in a drift tube. The nebulized eluent goes through a heated drift tube to evaporate the mobile phase. The temperature is optimized in function of the nature of the solute and the mobile phase. For low or non volatile compounds, the temperature of evaporation is increased to improve the detection.
 Detection of light diffusion using a Photomultiplier or a Photodiode
Detection of light diffusion using a Photomultiplier or a Photodiode
Measurement of light scattered by a stream of solid particles in suspension with gas entering a flow cell which includes a light source.
- Detection is obtained by the measure of the intensity of the scattered light.
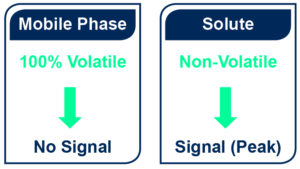
- A significant difference of volatility between the mobile phase and the compound is necessary. Caution must be consider for semi and highly volatile compounds detection as the signal is only generated by non-evaporated compounds.
- Both isocratic or gradient mode can be used
- There is no solvent restriction as long as it can be evaporated before detection and except: phosphate, sodium, sulphate, potassium, HCl and H2SO4 buffers that are forbidden.
- ELSD response has not a linear reponse with the concentration of the compound.
Caution:
The ESLD is a destructive method of detection for the sample.
As for purification recovery is the one of main goal, the lowest quantity possible of sample has to be sent into the ELSD.
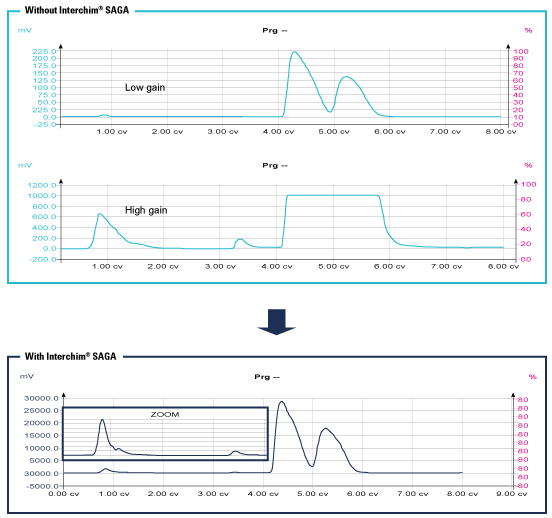
SAGA Function:
Interchim® developed with Sedere an innovative automatic gain (SAGA: Sedex Automated Gain Adjustement).
This technology adapt the gain to avoid saturation while continuing the detection of small quantity of products.
ELSD becomes almost unsaturable without an impact on sensitivity.
Application: Separation of 2 diastereo-isomers
Injection of 5mL (625mg of each compound)
Column: PF-15SIHP-F0025
Flow rate: 15mL/min
This advanced technology allows to detect all compounds regardless the quantity to purify.
Impurities can be seen even if a concentrated peak is close.
- Simplicity => automatic gain adjustment according to the sample load.
- Flexibility => manage both small quantity (2mg) and higher sample loading (up to 20%) within the same run.
- Confidence => numbers of class of compounds can be detected.
3. Mass Spectrometer Detectors (MS)
 A Mass Spectrometer measures the mass to charge ratio (m/z), so it converts sample compounds into ions. The ions fly under vacuum are sorted and separated according to their mass to charge ratio under the influence of an electrical and or a magnetic field. The detection system measures the amount of ions.
A Mass Spectrometer measures the mass to charge ratio (m/z), so it converts sample compounds into ions. The ions fly under vacuum are sorted and separated according to their mass to charge ratio under the influence of an electrical and or a magnetic field. The detection system measures the amount of ions.
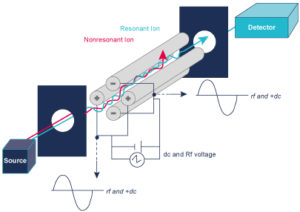
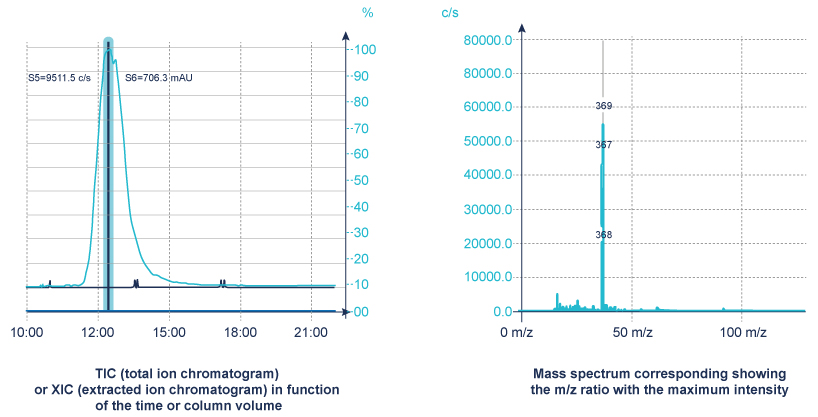
A mass spectrometer measures the spectrum over time (a sequence of spectra) to produce chromatograms
- TIC – Total Ion Chromatogram – adds all the masses together and shows how the entire mass spectrum varies with time – like a UV signal
- XIC (or EIC) – Extracted Ion Chromatogram – show how one mass varies with time, allows you to identify where your peak of interest elutes.
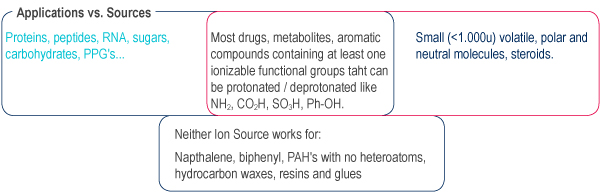
The MS detection is applicable to a broad range of organic compounds. As well as providing chromatographic data it definitively identifies the compound by confirming its mass.
The ionization of the compounds is made by a source.
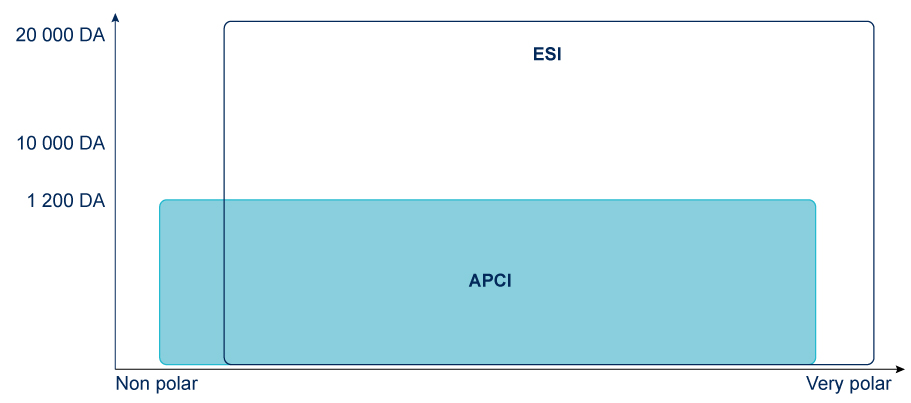
No Ion Source is universal, selection depends on the compound to be analyzed and the mass spectrometer type.
Two of the most common Ion Sources are Electro spray and Chemical Ionization and they can only be used with gas source (nitrogen).
APCI and ESI generally ionize by proton transfer:
- Acceptance of a proton to produce [M+H]+ in positive ion
- Abstraction of a proton from the analyte to produce [M-H]- in negative ion. Ionization may also occur by forming adducts with other species
- e.g. NH4+ (+18), Na+(+23), K+(+39), Methanol (CH3OHH+, +33), Acetonitrile (CH3CNH+, +42), Acetic Acid (CH3COOHH+, +61)
Electrospray is produced by applying a strong electric field to a liquid passing through a capillary electric field to a liquid passing through a capillary tube with a weak flux.
Desolvation by gas flow (N2) heating (100-300°C).
Ions are preformed in solution before nebulization
Ion Sources: APCI & ESI
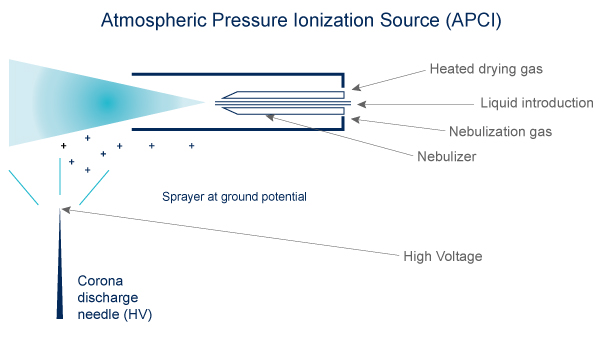 APCI
APCI
- Volatibility needed
- Compounds must be thermally stable
- Ions are formed in the gas phase
- The ions are singly charged
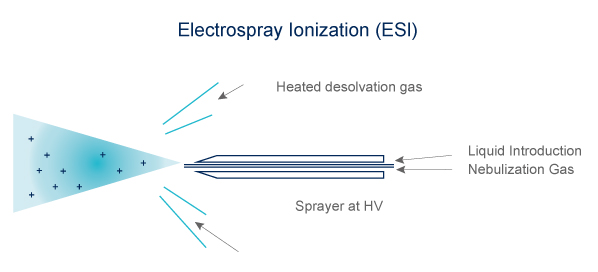 Electrospray (ESI)
Electrospray (ESI)
- Volatibility not mandatory
- Technique adapted to heat-labile compound
- Ions formed in solution
- Can form multi-charged ions
Why choose APCI for purification ?
- Better for neutral compounds than ESI
- Simpler spectra than ESI: more often the molecular M+H ion, less adducts, fragments dimers, No multiple charging
- Generally ‘Easy To do’: less sensitive to operating conditions that ESI
less solvent dependence as ionization occurs in the gas phase
less matrix suppression - Can accept higher sample concentrations
- More sensible than ESI: less noise at high flow rate ( >750 mL/min)
However, samples must be sufficiently volatile to be vaporized, & thermally stable to 130 – 150 °C
ESI might be considered for Reverse Phase and is essential for biological molecules.
The Mass spec. is a destructive method of detection for the sample.
As for purification recovery is the one of main goal, the lowest quantity possible of sample has to be sent into the MS.
Interchim® developed a Unique interface “Split & Dilution in a box” to avoid saturation of the MS spectrometer whatever the concentration of sample injected is. It does not generate additional backpressure even at high flow rate.
It is combined to a normalized scale of MS signals, UV, ELSD by Interchim software.
4. Triple Detection: UV-ELSD-MS
To Sum-up the detectors are compounds dependent.
| UV |
ELSD | MS | |
| Detection | Chromophore | Non volatile at the working temperature | Ionizable Gives an indication on the compounds structure |
To be sure to detect and collect all the compouds a combination of several detectors is advised.
A triple detection UV-ELSD-MS can be easily used with a puriFlash® system.
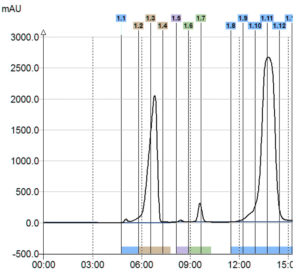
UV Signal
> Only 1 compound detected
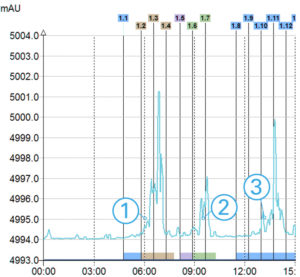
ELSD Signal
> 3 compounds detected
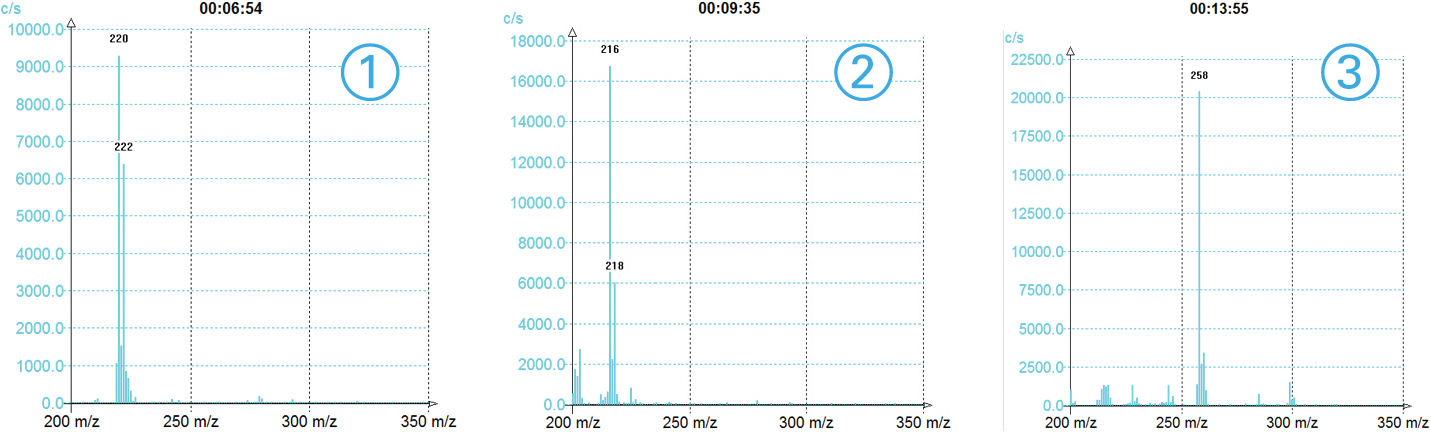
MS TIC Signal
>3 compounds detected
With information of the m/z for each peak
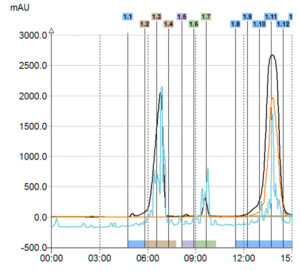
Triple Detection
In this example with only UV detection customer would have collected only one product instead of 3 with the triple detection. Moreover, with the MS he was able to identify his interest compounds.
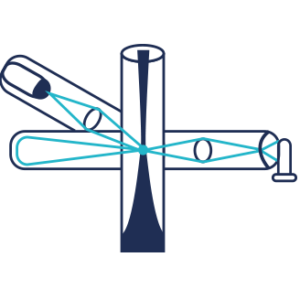
Refractive Index Detector (RI)
The RI detector measures the refractive index of an analyte relative to the solvent.
A light beam crosses a 2 compartments cell in which one is filled with the solvent and the other with the column effluent. So, this is a difference of refractive index of the 2 liquids which is measured.The greater the RI difference between sample and mobile phase, the larger the imbalance will become so the sensitivity will be higher. There is no detection if the refractive index of the compound is too close to the solvent refractive index.
Limits of Detection
RI detector is a pure differential instrument, and any changes in the eluent composition require the rebalancing of the detector. This factor is severely limiting RI detector application in the analyses requiring the gradient elution, where mobile phase composition is changed during the run.