Endotoxin is a major contaminant found in biologically active substances. Endotoxin, also called Lipopolysaccharide (LPS), is a cell membrane component of Gram-negative bacteria. A single E. coli cell contains about 2 million LPS molecules, which are released into the lysate during cell lysis, as occurs in production of most recombinant proteins. Many other water-born and fecal bacteria shed also LPS, contaminating gloves, labware, buffers and downstream products (cell culture media, solutions of purified substance or reagent).
The presence of endotoxin in drugs (pharma applications), synthetic ones as biosimilar and recombinant ones, can cause in host organisms pyrogenic reactions (fever), but also shivering, hypotension, adult respiratory distress syndrome, disseminated intravascular coagulation and toxic/endotoxin shock, tissue injury, and even death.
Endotoxin detection test is one of the most critical quality control tests required by the FDA for all drugs in their final formulation. Therefore, it is essential to remove endotoxin from human and animal parenteral drugs, biological products, and medical devices, from R&D step to clinical applications.
This article applies in particular to biotechnology applications, that received less attention than pharmaceutical field or mainly in this context.
1. Endotoxin in biotechnology
It is so essential to check for endotoxins (absence) and remove them from any samples or reagents that are used, or will be used downstream, with living cells, regardless of terminal sterilization procedures and/or aseptic practices (endotoxin concerns are only reduced). This applies in many fields : pharma, cosmetic, and even agro/food/ beverages [r], biomedical and health diagnostics (misinterpretation of results)).
Tips about endotoxins across biotech industriesBiomedical implants and in particular products intended for contact with cerebrospinal fluid have the most severe restrictions, being required to meet the most stringent US FDA standards for bacterial endotoxins. |
Endotoxin risks span many samples and reagents kinds for endotoxins-sensitive applications, and in each upstream step:
- in vitro techniques such as cell engineering, transfection of cells upstream cell culture;
- in culture media of cell cultures, in particular for the production of recombinant polypeptides/proteins or genes;
- in purified samples (i.e. buffers and fractions in chromatography);
- prior to administration into animal tissues/ bodies (ex-vivo and animal experimentations);
- in samples for endotoxins-sensitive cell-assays and cell-based assays, but also transfection.
2. Endotoxin removal
Endotoxin removal has been achieved using many techniques
- organic-water solvent extraction systems;
- aqueous two phase partitioning;
- ultrafiltration (size-based);
- size-exclusion chromatography;
- polymyxin B affinity removal;
- polylysine affinity removal;
- Ion-exchange chromatography (DEAE sepharose);
- some other ways can remove endotoxins in particular applications (not suitable for many processes), such as electrophoresis, detergents, ultracentrifugation, or enzymatic digestion of proteins.
Each method presents limitations: for most, the performance depends on sample/compound kind to be cleaned, through its ionic charge and size, but also its stability and biological activity. Some are applicable at small or large scale, some are quick, some are very labor-intensive, Upstream step and downstream requirements. For examples SEC chromatography can combine in some cases the purification step and the endotoxin removal (MW of compounds far from LPS) while compatible with downstream assays; poly-lysine eliminated toxicity issues associated using low quality polymixin B ligands and sodium deoxycholate buffers.
Technical tip – Endotoxin removalThe following first round try&check method is proposed:
|
3. Endotoxin Removal System
From DNA
For the rapid removal of endotoxins from previously purified DNA.
Endotoxins significantly reduce transfection efficiencies in endotoxin sensitive cell lines. Therefore, the removal of endotoxins from plasmid preparations is often necessary prior to the use of the DNA in such downstream applications.
Key Features
- Purify DNA with endotoxin levels of less than or equal to 0.1 EU/µg DNA
- Remove endotoxins from versatile DNA inputs
- Recover endotoxin-free DNA with > 90% recovery rate
- The recovered DNA is ready for transfections or other endotoxin-sensitive applications
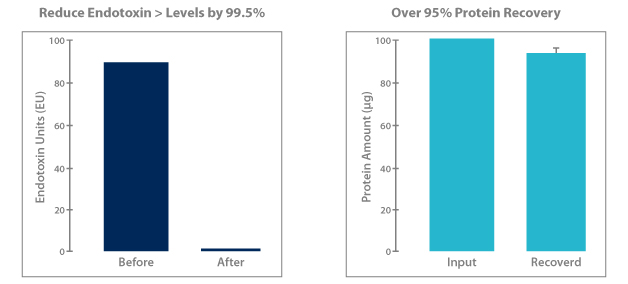
Norgen’s endotoxin removal kits efficiently reduce endotoxins to 0.1EU or less, providing plasmid DNA that is immediately ready for transfections or other endotoxin-sensitive applications. Norgen’s proprietary resin binds DNA while endotoxins, salts and other contaminants are washed away. The convenient spin column format can be completed in approximately 30 minutes with a typical recovery of DNA is >90% of the starting sample.
| Designation |
Part Number |
Quantity | Datasheet |
| Endotoxin Removal Kit (Mini) – For DNA | 22700 | 25 preps | |
| Endotoxin Removal Kit (Midi) – For DNA | 52200 | 10 preps | |
| Endotoxin Removal Kit (Maxi) – For DNA | 21900 | 4 preps |
From proteins, peptides, and antibodies
 |
Endotoxins can initiate a strong immune response in mammalian hosts, resulting in systemic inflammatory response syndrome (SIRS) and/or sepsis. Endotoxins are often difficult to remove from solution due to the high variability of their molecular weights. In addition, endotoxins are relatively stable and insensitive to changes in temperature and pH.
Ligands with affinity to endotoxins can be coupled to an anion exchange system to increase the endotoxin binding strength. ToxinEraser Endotoxin Removal System is based on an affinity matrix of modified polymyxin B (PMB), with a high binding capacity (> 2 000 000EU/mL). It allows for efficient endotoxin removal from samples such as proteins, peptides, and antibodies. The final endotoxin level can be decreased to as low as 0.1EU/mL* with repeated use of ToxinEraser Endotoxin Removal Resin. . |
*Final removal efficiency may vary depending on the sample type/source.
Key Features
- High binding capacity at least 2 000 000EU/mL (CV)
- High Recovery Yield: >90% with minimized sample loss
- High stability and high removal efficiency
| Designation | Part Number |
Quantity | Datasheet |
| ToxinEraserTM Endotoxin Removal Kit | L00338 | 1 Kit |
Endotoxin Detection
ToxinSensor Endotoxin Detection System uses FDA approved LAL (Limulus Amebocyte Lysate) testing methods to achieve a fast and highly sensitive endotoxin assay. The Chromogenic LAL Endotoxin Assay kit can quantitatively detect endotoxin in a broad range (0.005-1EU/mL). The Gel Clot Endotoxin Assay kit is a fast qualitative test showing a positive or negative result.
ToxinSensor Endotoxin Detection System
To ensure accurate results with rapid endotoxin assay method!
- Delivered with ready-to-use reagents and materials
- Strong linearity and reproducibility
- Guaranteed high-sensitivity: 0.005EU/mL
ToxinSensor Endotoxin Assay Kits can be widely used in in vitro end-product endotoxin tests, including those for human and animal parenteral drugs, biological products, and medical devices.
Chromogenic LAL Assay
The kit utilizes a modified Limulus Amebocyte Lysate and a synthetic color producing substrate to quantitatively detect endotoxin chromogenically in a broad range (0.005-1EU/mL).
| Designation | Part Number |
Quantity | Datasheet |
| ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit | L00350 | 1 Kit (32 rxns) | |
| ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit | L00350C | 1 Kit (16 rxns) |
Chromogenic LAL Assay
The kit utilizes a modified Limulus Amebocyte Lysate and a synthetic color producing substrate to quantitatively detect endotoxin chromogenically in a broad range (0.005-1EU/mL).
| Designation | Part Number | Quantity | Datasheet |
| ToxinSensor™ Gel Clot Endotoxin Assay Kit | L00351 | 1 Kit |
ToxinSensor Single Test Kit
The kit is designed to be a qualitative In Vitro end-point endotoxin test for human and animal parenteral drugs, biological products, and medical devices.
Limul Amebocytuse Lysate (LAL) as supplied is to be reconstituted with sample or control directly. There is no need to reconstitute the system with LAL Reagent Water before use. After incubation for 1 hour, and in the presence of endotoxin, gelation occurs; in the absence of endotoxin, gelation does not occur.
A series of ToxinSensor™ Single Test Kits is supplied with different sensitivity (0.015EU/mL, 0.03EU/mL, 0.06EU/mL, 0.125EU/mL and 0.25EU/mL).
When using the ToxinSensorTM Single Test Kit, you just need to add 200µL of sample or control to LAL, then, wait for the required incubation time to allow for gel to be formed.
| Designation | Part Number | Quantity | Datasheet |
| ToxinSensor™ Single Test Kit | L00448-40 | 1 kit (40 assay) 0.03EU/mL | |
| ToxinSensor™ Single Test Kit | L00448-20 | 1 kit (20 assay) 0.03EU/mL | |
| ToxinSensor™ Single Test Kit | L00449-20 | 1 kit (20 assay) 0.06EU/mL | |
| ToxinSensor™ Single Test Kit | L00449-40 | 1 kit (40 assay) 0.06EU/mL | |
| ToxinSensor™ Single Test Kit | L00450-20 | 1 kit (20 assay) 0.125EU/mL | |
| ToxinSensor™ Single Test Kit | L00450-40 | 1 kit (40 assay) 0.125EU/mL | |
| ToxinSensor™ Single Test Kit | L00451-20 | 1 kit (20 assay) 0.25EU/mL | |
| ToxinSensor™ Single Test Kit | L00451-40 | 1 kit (40 assay) 0.25EU/mL |
Accessory Products
| Designation | Part Number | Quantity | Datasheet |
| ToxinEraser™ Endotoxin Removal Resin | L00402 | 1mL | |
| ToxinSensor™ Endotoxin-free Tubes | M01072-10 | 10 Tubes | – – – |
| ToxinSensor™ Endotoxin-free Pipette Tips (1mL, Blue) | M01063 | 1PK of 6 tips | – – – |
| ToxinEraser™ Regeneration Buffer | M01053 | 125mL | – – – |
| ToxinEraser™ Equilibration Buffer | M01054 | 125mL | – – – |
Endotoxin free reagents
Search our low endotoxin and free endotoxin.
- Bovine Albumin, Low endotoxin, 30% solution #C71151
- Bovine Serum Albumin, Low endotoxin # C71141
- Bovine Serum Albumin, VERY LOW Endotoxin #C7114A
Consult datasheet.
Know more:
- Contact us: interbiotech@interchim.com