In December 2019 a 7th strain of coronavirus (CoV) capable of infecting humans emerged. called SARS-Cov-2 and causing Covid-19 disease (for Coronavirus disease 2019), this virus spread rapidly throughout the world. Like its congeners, it uses the membrane receptor ACE2 to entry into the host cells. A recent study published in Cell review (1), the result of an international collaboration, focuses on this receptor and highlights how SARS cov-2 uses to its profit the production of interferon, which is supposed to slow down its multiplication, in order to better infect the cells of its host. In addition, using single-cell RNA-sequencing (scRNA-seq), these same researchers have identified the tissues most sensitive to the coronavirus.
| Of the 7 coronavirus (CoV) strains capable of infecting humans, only SARS-CoV, MERS-CoV and currently SARS-CoV-2 have caused pandemics. The other strains HCoV-OC43, HCoV-229E, HCoV-NL63 and HCoVHKU1 are not highly pathogenic and cause only mild respiratory disease. |
Reminder
| In the early 2000s, the study of the infiltration mechanism of SARS-COV and HCoV-NL63 coronaviruses led to the identification of ACE2 as a entry for target cells; the latter binds to the coronavirus skype protein. This interaction is mediated by the RBD domain of the spyke protein, and is considered the pivotal event in the membrane fusion process. It has been shown that SARS-COV-2 also uses ACE2 as a receptor to entry into host cells. In this key step, a third actor plays a crucial role, the enzyme TMPRSS2, which is a protease that facilitates the entry of the virus into the cell. |  |
Identification of most sensitive tissues to coronavirus:
Angiotensin converting enzyme 2 (ACE2) is an essential regulator of cardiac function. Studies have shown that this membrane receptor is expressed on the surface of epithelial cells in the heart, lungs, kidneys and gastrointestinal tract.
Thanks to the “single cell RNA sequencing” technique, a detailed study of the genetic profiles of the cells studied in this publication made it possible to identify those that possess the mRNAs of the receptor ACE2 genes and the TMPRSS2 enzyme, which are therefore considered to be the cells most sensitive to coronavirus. These three populations are as follows:
- Lung type II pneumocytes,
- Ileal absorptive enterocytes,
- Nasal goblet secretory cell.
Influence of Interferons on ACE2 Synthesis
After characterizing the tissues most likely to be infected with SARS-Cov-2, the researchers investigated the influence of interferons on the level of ACE2 expression. Deregulation of ACE2 was observed in lung damages caused by RSV (respiratory syncytial virus) and avian influenza (H5N1) infections. Administration of ACE2-Fc fusion protein was shown to improve respiratory symptoms in animal models.

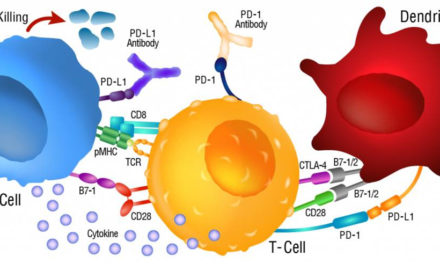
Virus-infected host cells inhibits protein synthesis and DNA replication in virus-infected cells increases the expression of MHC class I and antigen presentation by all cells Activates NK cells to destroy virus-infected cells. |
Infection of cells results in the synthesis of proteins that interfere with viral replication and allow uninfected cells to resist viral infection. Because of their anti-viral function, these proteins have been called Interferons. Interferons (IFNs) are classified into 3 types: type I (including the interferons α and β) and type III are involved in innate immunity, type II consisting of the interferon ɣ is synthesized by T cells and appears after induction of the adaptive immune response. Note that interferon α includes a whole family of related proteins. It has been shown that interferons (in particular interferon α) stimulate the expression of the gene ACE2 in the most sensitive tissues. Thus SARS-Cov 2 uses the host’s defence mechanisms to its profit, as entering SARS-Cov cells triggers the synthesis of iFNα which, instead of slowing it down, will accelerate its infiltration by multiplying the receptor ACE2. |
Therapeutic approaches
The Cov-2 SARS outbreak triggered a strong reactivity and collaboration from the scientific community. Various bioactives have been tested against the infection : protease inhibitors such as Lopinavir #PO407S or Ritonavir #LSI940, RNA-dependent RNA polymerase (RdRp) inhibitors such as Remdesivir (#B60DF0), inhibitors of the ACE / Renin pathway such as Losartan (#XLQ590) and the EK1 peptide. Others are aimed at preventing inflammatory crisis (cytokine storm). including antimalarial agents like chloroquine.
Positive results from the administration of tocilizumab offer hope to any patient suffering from severe forms of Covid-19. Tocilizumab is a therapeutic monocloclonal antibody antagonist of Il-6, a cytokine that plays a major role in the acute phase of inflammation and thus ultimately in the cytokine storm that may ensue.
The Germans are now offering Camostat mesilate (#B60DF0), already approved in Japan. This drug is a TMPRSS2 protease inhibitor and therefore aims to block the entry of the virus into cells.
In the perspective of a vaccine, encouraging results on the absence of reinfection have been obtained by Chinese teams on macaques. This recent study involving a very small number of primates remains to be confirmed. Still in China, results are expected following clinical trials carried out in March. The race for the vaccine has also begun in Europe, with clinical trials will begin in the United Kingdom and Germany. The development of a vaccine is expected to take a minimum of 12-18 months.
In the absence of a curative treatment, and in order to limit the spread of the virus while supporting all healthcare personnel, the best thing to do is to respect the barrier gestures with, in the first instance, social (and physical) distancing, regular hand washing, and the systematic wearing of masks in public and closed places. It is by being united that we will succeed.
Interchim® support scientific research by offering a complete panel of ACE2 proteins and antibodies from its best product ranges.
Know more:
(1) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues
Carly G. K. Ziegler1,2,3,4,5,6*, Samuel J. Allon2,4,5,7,*, Sarah K. Nyquist2,4,5,8,9,*, Ian M. Mbano10,11,*, Vincent N. Miao1,2,4,5, Constantine N. Tzouanas1,2,4,5, Yuming Cao12, Ashraf S. Yousif4, Julia Bals4, Blake M. Hauser4,13, Jared Feldman4,13,14, Christoph Muus5,15, Marc H. Wadsworth II2,3,4,5,7, Samuel W. Kazer2,4,5,7, Travis K. Hughes1,4,5,16, Benjamin Doran2,4,5,7,17,18, G. James Gatter2,4,5, Marko Vukovic2,3,4,5,7, Faith Taliaferro5,18, Benjamin E. Mead2,3,4,5,7, Zhiru Guo12, Jennifer P. Wang12, Delphine Gras19, Magali Plaisant20, Meshal Ansari21,22,23, Ilias Angelidis21,22, Heiko Adler22,24, Jennifer M.S. Sucre25, Chase J. Taylor26, Brian Lin27, Avinash Waghray27, Vanessa Mitsialis18,28, Daniel F. Dwyer29, Kathleen M. Buchheit29, Joshua A. Boyce29, Nora A. Barrett29, Tanya M. Laidlaw29, Shaina L. Carroll30, Lucrezia Colonna31, Victor Tkachev17,32,33, Christopher W. Peterson34,35, Alison Yu17,36, Hengqi Betty Zheng36, Hannah P. Gideon37,38, Caylin G. Winchell37,38,39, Philana Ling Lin38,40,41, Colin D. Bingle42, Scott B. Snapper18,28, Jonathan A. Kropski43,44,45, Fabian J. Theis23, Herbert B. Schiller21,22, Laure-Emmanuelle Zaragosi20, Pascal Barbry20 Alasdair Leslie10,46, Hans-Peter Kiem34,35, JoAnne L. Flynn37,38, Sarah M. Fortune4,5,47, Bonnie Berger9,48, Robert W. Finberg12, Leslie S. Kean17,32,33, Manuel Garber12, Aaron G. Schmidt4,13, Daniel Lingwood4, Alex K. Shalek1-8,16,33,49,#, Jose Ordovas-Montanes5,16,18,49,#, HCA Lung Biological Network