Origins & evolution of chromatography
The term “chromatography” originated in 1906 thanks to Russian botanist Mikhail Tswett. In 1901, he washed an organic solution of plant pigments through a vertical glass column packed with an adsorptive metal. He discovered that the pigments separated into a series of colored bands on the column, divided by regions entirely free of color.
In 1930, chemists Richard Kuhn and Edgar Lederer used this technique to separate different biologically materials. Since that time, the technique has advanced rapidly and column chromatography is now used widely in many different forms. The column itself has also been refined over the years, according to the type of chromatography, but fulfils the same essential separating function in all forms of column chromatography.
In 1964, the American chemist J. Calvin Giddings refined liquid chromatography to achieve separations of different molecules. This was the origin of the technique now known as High Performance Liquid Chromatography (HPLC), and relied on very small particles size in small diameter columns.
From the mid 80’s a number of scientists’ as Verzele & Dewaele, Bildlingmeyer, Unger, … published articles dedicated to Preparative Liquid Chromatography on the technique itself, the columns and instruments technology.
Preparative liquid chromatography
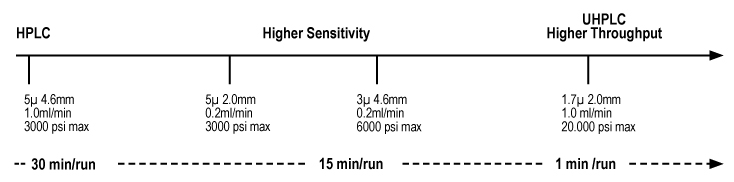
Analytical liquid chromatography
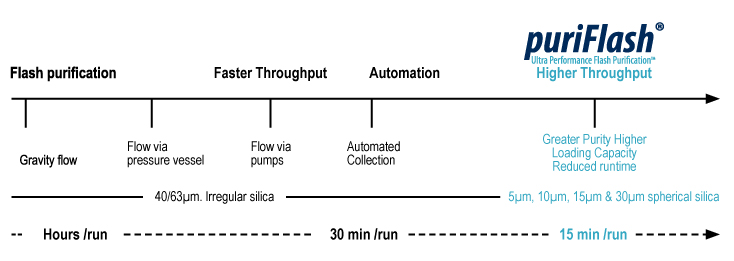
Since 1995, Interchim® is an essential actor of the purification market.
In 2008, Interchim® launched puriFlash® a range of advanced automated instruments and consumables supported by the Ultra Performance Flash Purification concept who has revolutionized the purification practices.
Versatile, these systems allow chemists and bio-chemists to work with Flash cartridges and Preparative columns on a single device.
 |
In combination with more than 50 different chemistries, 12 puriFlash instruments are available to perform purification of small Organics Molecules, Natural Products, Peptides, and Proteins. |
Liquid Chromatography principle
Liquid Chromatography is a separation technique.
It can be dedicated to identify and quantify compounds present in a mixture, this is the analytical mode. Very attractive when the goal is to get isolated a pure product from a more or less complex mixture, this technique is then called preparative liquid chromatography and seems to be today the most popular way for purification.
Liquid chromatography manages compromise between multiple parameters and primarily stationary phase, eluents and compounds of interest.
Compounds are eluted by a liquid mobile phase (eluent) in contact with a stationary phase (fixed). The migration speed of the species contained in the sample depends on the interactions with the stationary phase (adsorption or desorption phenomenon), the mobile phase or their solubility and polarity.
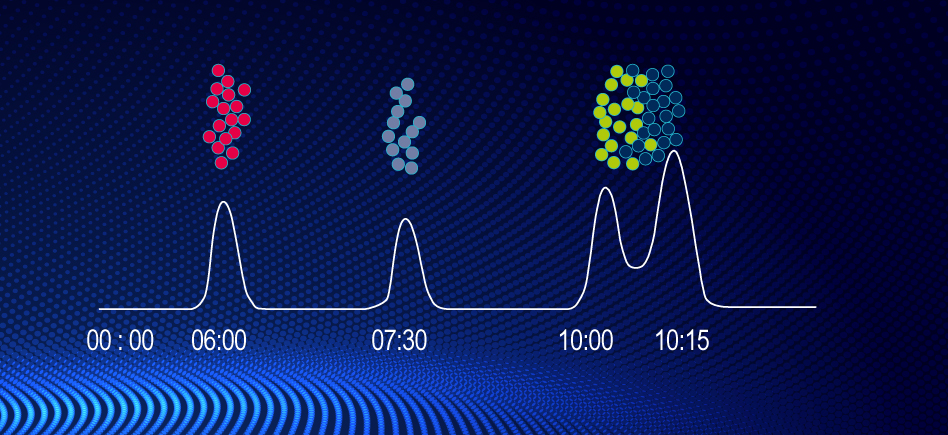
Exemple: 4 groups of products in different quantities
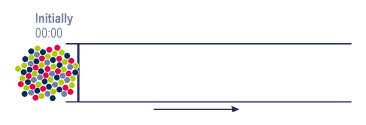 |
Objective : separate the components of a mixture containing different molecules in order to qualify (identify, quantify) and/or purify them. |
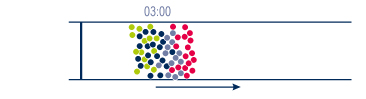 |
In the mixture or the raw simple, each group of molecules will have a particular behaviour while going through the column. This behaviour is related to their own reaction with the eluent & the stationary phase. |
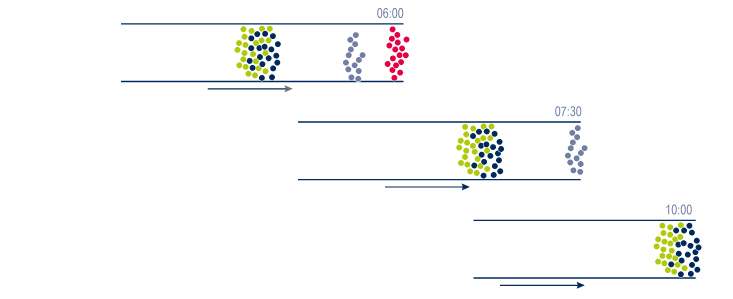
| What we see on arrival => | 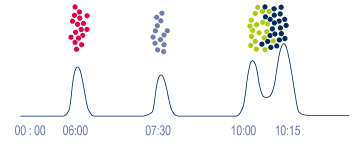 |
Know more:
- Conctact us: interchrom@interchim.com
- Discover our product range dedicated to liquid chromatography
- Discover all our preparative systems