Introduction to the boronic acids
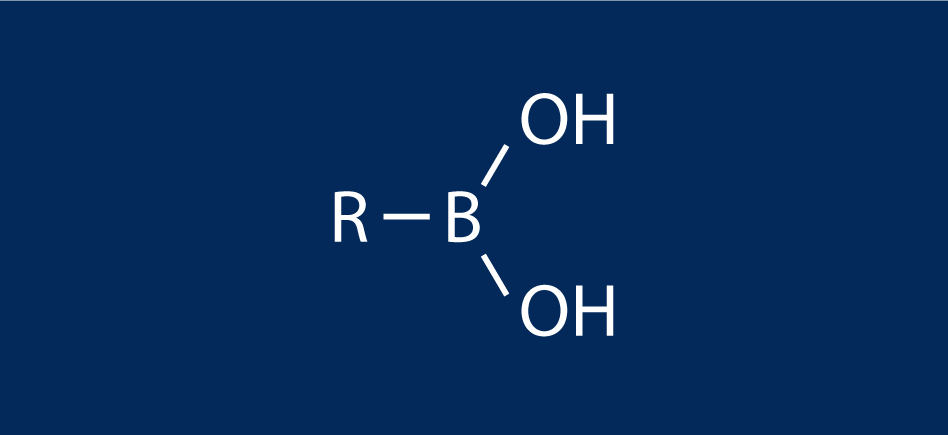
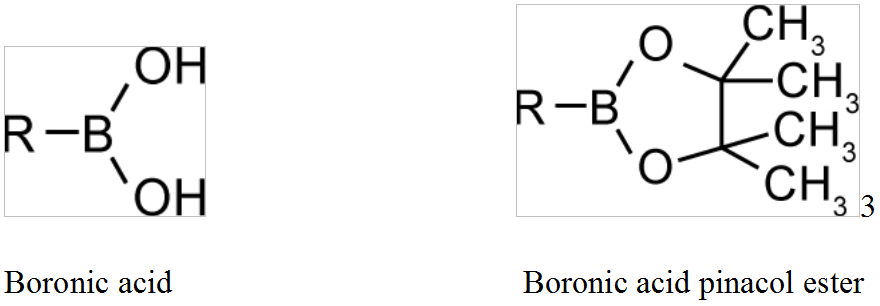
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes.
Boronic acids : the function
Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc. (molecules with vicinal, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol, amine, carboxylate)). The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7.
In what situation do we use boronic acids ?
They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the Suzuki coupling. A key concept in its chemistry is transmetallation of its organic residue to a transition metal.
R-B(OH)2 + R’-X ►R-R’
Suzuki–Miyaura reaction
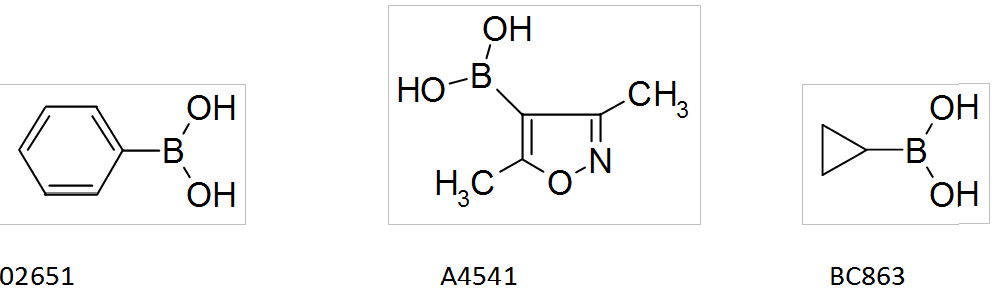
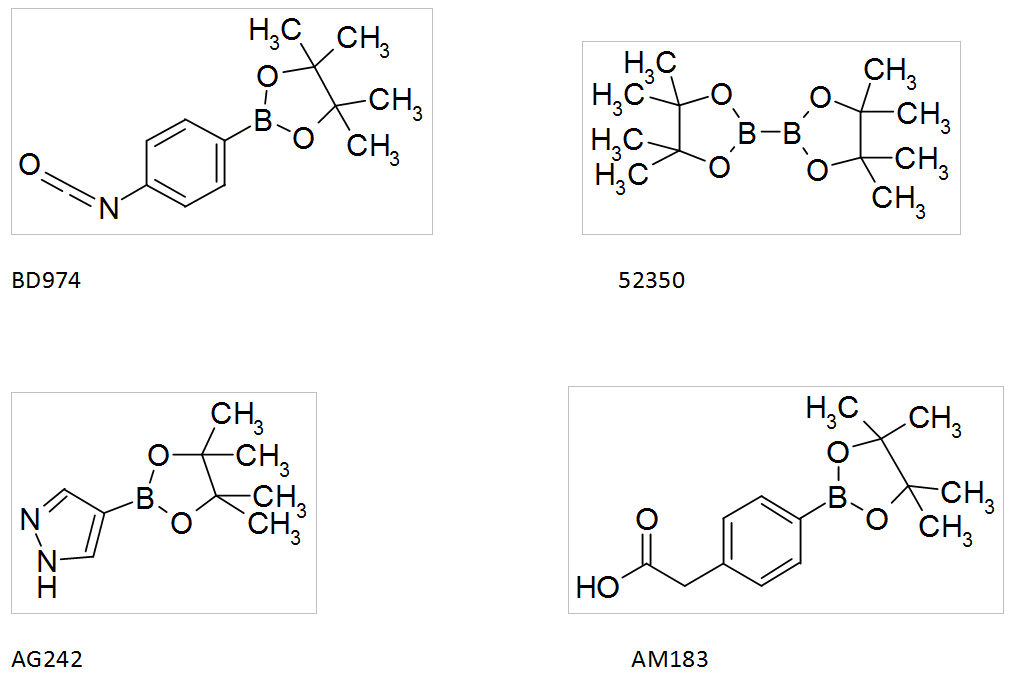
Boronic acids by Interchim
Interchim offers a wide range of boronic acids that you can find in the catalog SYNHTESIS INTERMEDIATES, such as:
Know more :
- Download the list of all the Interchim boronic acids
- Don’t hesitate to contact interfine@interchim.fr for any request concerning the price