PolyHistidine Tags (His Tags) are a popular addition to recombinant proteins to facilitate protein purification and detection. His Tags are commonly used in preference to other tags due to the availability of inexpensive purification resins such as NiNTA. Anti-His Tag antibodies enable the detection of the tagged proteins allowing screening of expression. The poly-His Tag is usually formed of 6 histidine residues but may be between three and ten, usually at the C- or N-terminus of the recombinant protein. Here we demonstrate the utility of Jackson ImmunoResearch’s Anti-His Tag antibodies for the detection of His-Tagged proteins by both Western blotting and ELISA.
| Description | P/N | Cond. |
| AffiniPure Rabbit Anti-His Tag | 300-005-240 | 0.1mg |
| Biotin-SP (long spacer) AffiniPure Rabbit Anti-His Tag | 300-065-240 | 0.1mL |
| Alkaline Phosphatase AffiniPure Rabbit Anti-His Tag | 300-055-240 | 0.1mL |
| Peroxidase AffiniPure Rabbit Anti-His Tag | 300-035-240 | 0.1mL |
| Alexa Fluor® 488 AffiniPure Rabbit Anti-His Tag | 300-545-240 | 0.1mg |
| Alexa Fluor® 790 AffiniPure Rabbit Anti-His Tag | 300-655-240 | 0.1mg |
| Alexa Fluor® 680 AffiniPure Rabbit Anti-His Tag | 300-625-240 | 0.1mg |
| Alexa Fluor® 647 AffiniPure Rabbit Anti-His Tag | 300-605-240 | 0.1mg |
Confidently screen His-Tagged proteins in cell lysates by Western blot
Protein expression screening of His-Tagged recombinant proteins (constructs) from cell lysates is a popular application of Anti-His Tag antibodies. Anti-His Tag antibodies allow the detection of both carboxy- and amino- terminally His-Tagged proteins. Figure 1 demonstrates the detection of a recombinant His-Tagged protein from mammalian, insect, and bacterial cell lysates.
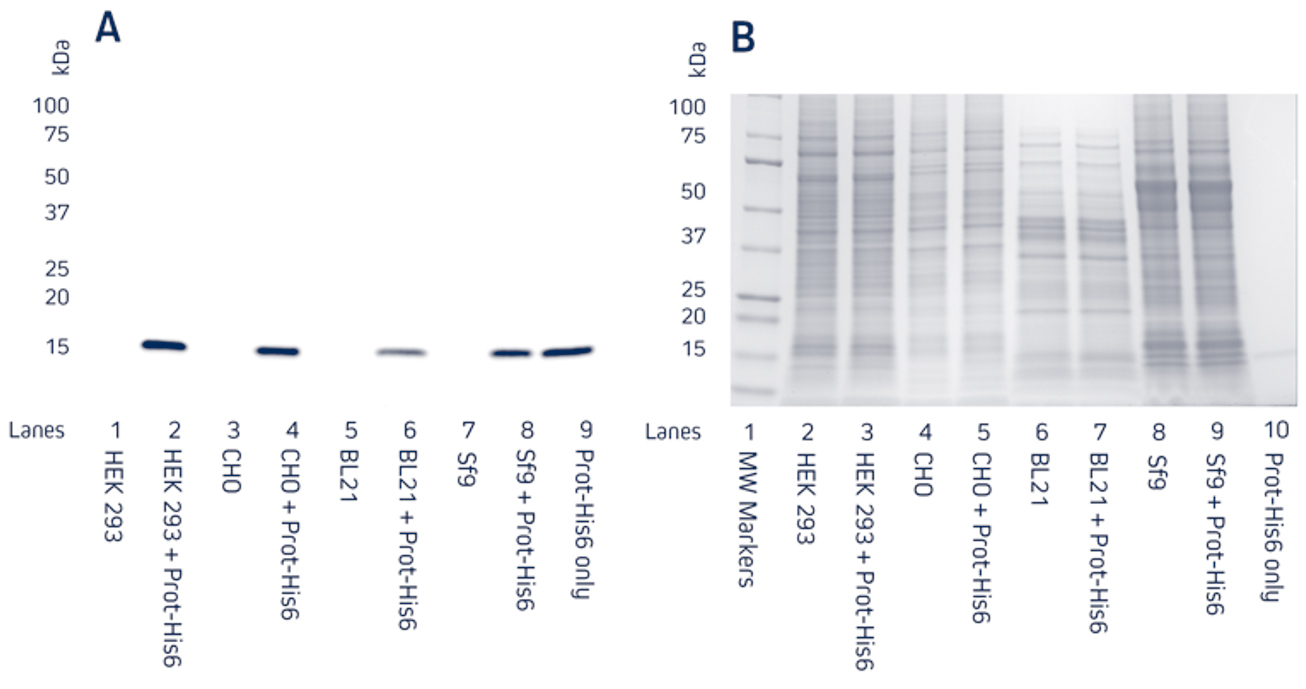
Figure 1. Detection of His-Tagged proteins in a range of cell lysates.
C-terminally His-Tagged protein was added at 100ng to the cell lysates. Cell lysates of HEK 293, CHO, BL21 E. coli, and Sf9 insect cells were reduced and boiled for 5 minutes at 100°C and loaded at 9μg/well in tandem SDS gels.
A. Western blot. Gels were electroblotted onto nitrocellulose membrane, blocked with 5% BSA in PBS/0.2% Tween 20, and probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at 1:20K dilution and visualized using a digital imager.
B. Gels were stained with Coomassie.
Use conjugated Anti-His Tag antibodies directly for single-step Western blotting
Jackson ImmunoResearch’s Anti-His Tag antibodies can be used directly using a Horseradish Peroxidase conjugate. Figures 2 and 3 demonstrate that Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag antibody can be used across a range of dilutions and that sensitive detection of different His-Tagged proteins can be achieved. The sensitivity of the Western blot assay can be enhanced by performing the assay indirectly, using a conjugated secondary antibody to achieve increased signal shown in the following section.
 |
 |
|
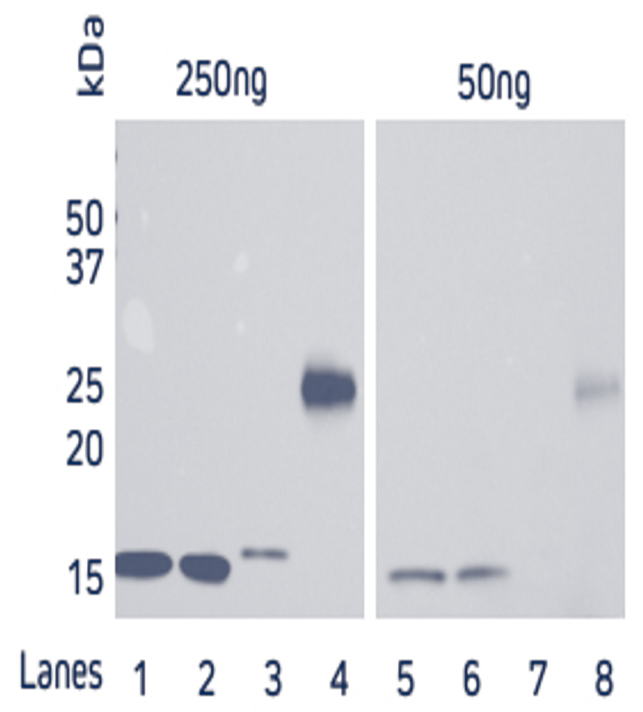
Figure 2. Western blot showing the detection of His-Tagged protein at two concentrations of HRP Rabbit Anti-His Tag antibody. A recombinant His-Tagged protein was reduced and boiled for 5 minutes at 100°C and loaded in two sets of 50, 100 and 250ng/well in an SDS gel. The gels were electroblotted onto a nitrocellulose membrane, which was then halved and each set of samples probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at either 1:5K or 1:50K. The resulting bands were visualized using a digital imager, the 1:5K blot was exposed for 3 seconds, and the 1:50K blot was exposed for 21 seconds. |
Figure 3. Direct Western blot showing the limit of detection for four recombinant His-Tagged proteins by HRP Rabbit Anti-His Tag antibody. Four recombinant His-Tagged proteins were reduced and boiled for 5 minutes at 100°C and loaded into an SDS gel in two sets of 50 and 250ng/well. Lanes 1, 2 and 4 have C-terminally tagged proteins while Lane 3 contains an N-terminally tagged protein. The gels were electroblotted onto a nitrocellulose membrane, and probed with Jackson ImmunoResearch’s HRP Rabbit Anti-His Tag (300-035-240) at a dilution of 1:20K. The resulting bands were visualized using a digital imager. |
Enhance sensitivity by using Jackson ImmunoResearch’s Anti-His Tag antibody in combination with Anti-Rabbit conjugates
Signal enhancement can be achieved by indirect detection of the His Tag using an Anti-His Tag antibody followed by a conjugated Anti-Rabbit antibody such as an HRP Goat Anti-Rabbit secondary antibody. Figure 4 shows that sensitivity can be improved by using the indirect two-step method (B) in comparison to direct detection (A).
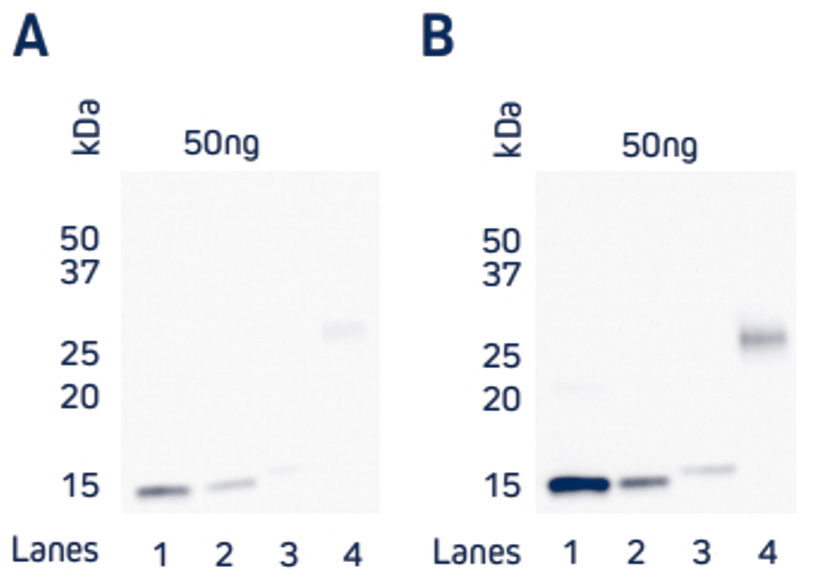
Figure 4. Comparison of direct and indirect Western blot detection of four recombinant His-Tagged proteins.
Samples
Lane 1: Protein 1 (C-term His Tag) – 50 ng
Lane 2: Protein 2 (C-term His Tag) – 50 ng
Lane 3: Protein 3 (N-term His Tag) – 50 ng
Lane 4: Protein 4 (C-term His Tag) – 50 ng
His-Tagged proteins were boiled and reduced in SDS-PAGE loading buffer containing 5% beta-mercaptoethanol (BME) and loaded at 50ng/well as detailed above. Gels were electroblotted onto nitrocellulose and blots were probed. A) Direct: HRP Rabbit Anti-His Tag (300-035-240) at a dilution of 1:20K. B) Indirect: Rabbit Anti-His Tag (300-005-240) at a dilution of 1:5K followed by HRP AffiniPure Goat Anti-Rabbit IgG (H+L) (min X Hu, Ms, Rat Sr Prot) (111-035-144) at a dilution of 1:20K. Blots were visualized with a digital imager.
Anti-His Tag antibody for ELISA
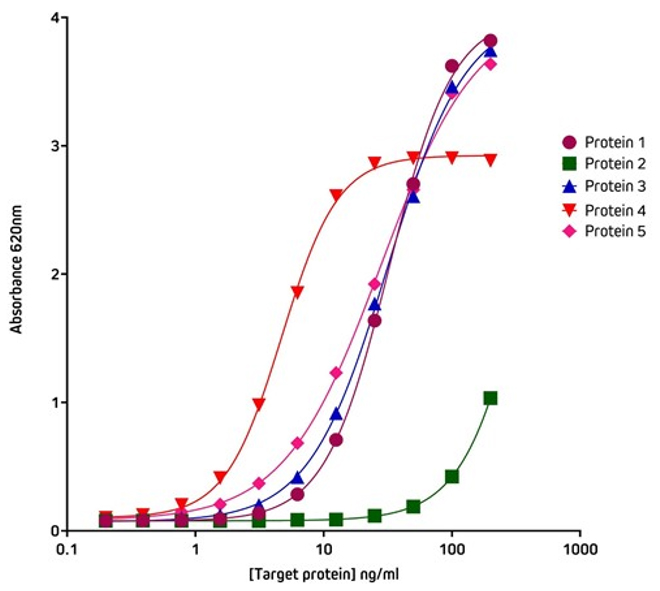
Figure 5 shows the variation in binding characteristics of a range of recombinant His-Tagged Alpaca VHH antibodies isolated from an activity screen against antigen X and analyzed by ELISA. Variations in binding affinity of the different nanobodies can clearly be discerned and lead candidates selected.
Figure 5. Detection of His-Tagged proteins with HRP Rabbit Anti-His Tag (300-035-240) by ELISA.
Coating Antigen: Antigen X was coated onto the plate at 1 μg/well (100µl, 10μg/ml). The plate was blocked with 1% Bovine Serum Albumin (IgG-Free, Protease-Free) (001-000-162) in PBST.
Sample: 5 individual purified His-Tagged recombinant Alpaca VHH Anti-Antigen X antibodies (Protein 1-5), loaded as serial dilutions of 20 ng (100µl @ 200ng/ml); 1:2 across the plate.
Detection Antibody: HRP Rabbit Anti-His Tag (300-035-240) diluted 1:20,000 (100μl per well).
Plate incubated with TMB for 30 minutes at room temperature and read at 620nm using a plate reader.
Screening ELISA using His Tag capture
The His-Tag offers another method of protein capture that may be useful when working with proteins subject to conformational sensitivity, allowing the protein to be elevated above the plate surface making more epitopes accessible to immunoreagents. Another application for the Anti-His Tag antibody is in situations where there is limited target protein-specific antibody availability, but the assay requires two different antibodies to generate the sandwich format. By capturing with the His Tag, target protein-specific antibodies can be used for detection in complex with conjugated species-specific secondary antibodies, allowing optimal signal amplification. His Tag capture also provides antibody-mediated, rapid and consistent binding of antigen to the ELISA plate.
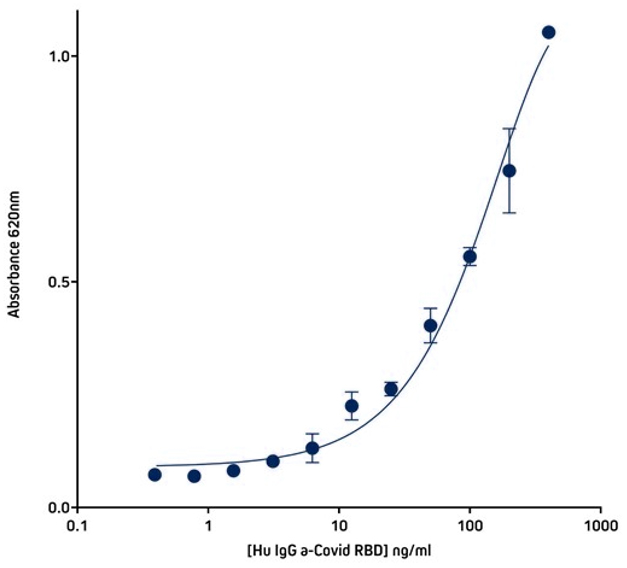
Figure 6. Detection of His-Tagged protein using Rabbit Anti-His Tag (300-005-240) by indirect Sandwich ELISA.
Capture Antibody: AffiniPure Rabbit Anti-His Tag (300-005-240) coated at 1μg/well (100μl, 10μg/ml). The plate was blocked with 1% Bovine Serum Albumin (IgG-Free, Protease-Free) (001-000-162) in PBST
Sample: His-Tagged SARS-CoV-2 spike protein RBD (Genscript – Z03483-100) loaded at 20ng/well (100μl @ 200ng/ml).
Detection Antibody 1: Human IgG anti-SARS-CoV-2 Spike S1 Antibody (Genscript A02038). 40 ng (100µl @ 400ng/ml) in first well; serially diluted 1:2 across plate.
Detection Antibody 2: HRP Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Ms, Rb Sr Prot) (109-035-170) diluted 1:20,000 (100μl per well).
Plate incubated with TMB for 30 minutes at room temperature and read at 620nm using a plate reader.
Know more:
- Do not hesitate to consult us to benefit from our years of experience in immunoassays, immunoreagents and ELISA kits
- Consult an extract from our BioSciences catalog (in french only)