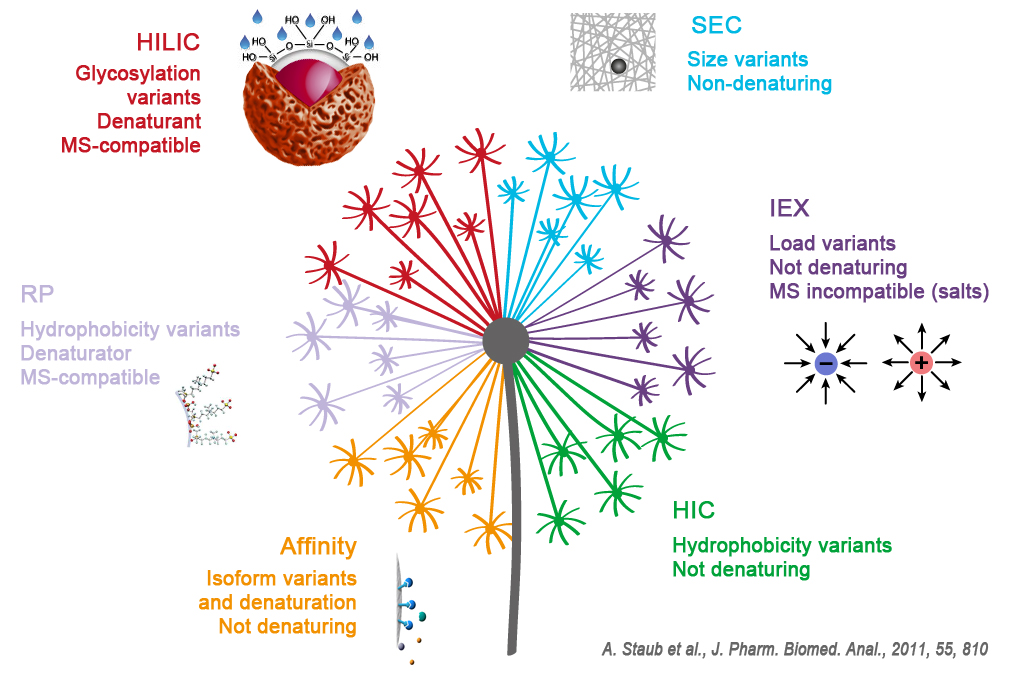
On a previous article we described all the existing technics dedicated to the analysis & purification of proteins.
This article aims at focusing on a specific technic: Ion Exchange Chromatography (IEX)
Two kinds of IEX are existing: CIEX = Cation IEX, AIEX = Anion IEX
- Cation IEX: cation exchange, exchange of positively charged molecules
- Anion IEX: anion exchange, exchange of negatively charged molecules
Ion exchange chromatography:
Ion exchange chromatography (IEX) separates proteins with differences in surface charge to give high-resolution separation with high sample loading capacity. The separation is based on the reversible interaction between a charged protein and an opposingly charged chromatography resin.
As binding kinetics for IEX are fast, ion exchange chromatography resins can be used at high flow rates.
IEX is used when you need a protein with very high purity (3 steps purification) and this for any kind of proteins, antibody, recombinant proteins and untagged proteins.
IEX – Principle
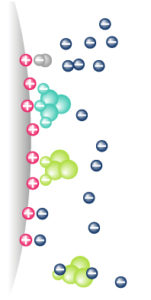 |
Molecules are separated following their global surface charge It depends on pH & ionic strength (conductivity) of the mobile phase (eluent) |
 |
Advantages
- High flow rate, highly selective
- High capacity
- Very high yield
- Sample concentration
Disadvantages
- pH dependent
- Proteins are in a high ionic strength buffer at the end of the purification (large salt concentration)
- The sample must be loaded at a low ionic strenght
- Presence of clusters of (+) charged residues can lead to the link of a (-) net charged protein on a cation exchange resin and vice versa
- The link profile can be modified with small pH modifications
- The charge of the buffer must be of the same sign as the IEX mode in order to not interfere with the molecules to purify (If anion exchange, buffer must be negatively charged and inversely for cation exchange)
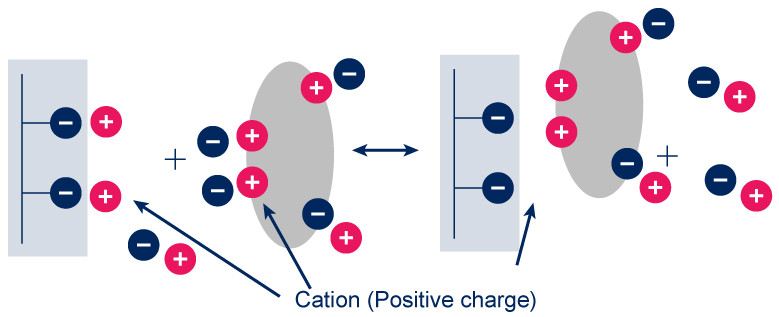
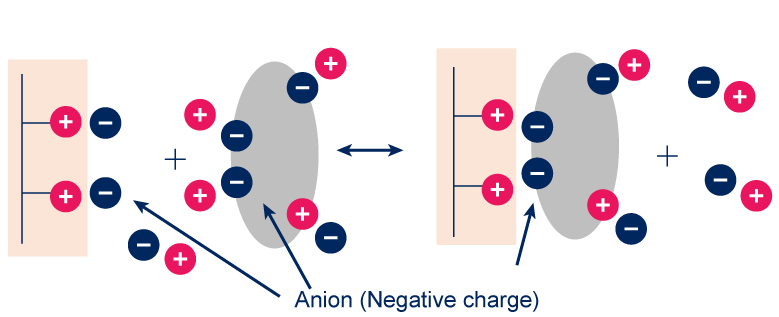
Ion exchange resins contain charged groups
These may be acidic in nature (in which case the resin is a cation exchanger – exchange
of positively charged molecules-) or basic (in which case it is an anion exchanger – exchange
of negatively charged molecules-). Cation and anion exchangers may be broken down further into weak & strong exchangers (reflecting binding affinity).
Usually, samples are loaded under low ionic strength conditions & bound material is eluted using either a step or gradient elution of buffer with higher ionic strength.
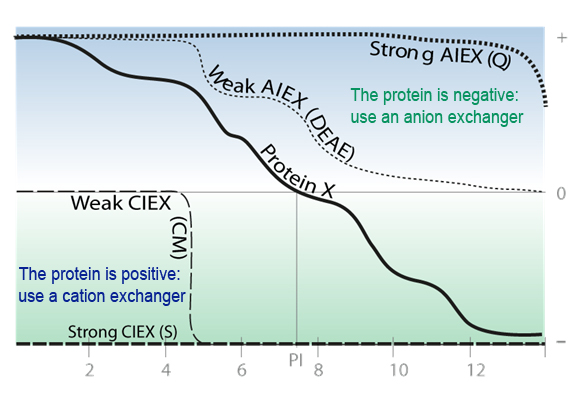
Generally speaking, a protein will bind to a cation exchange resin if the buffer pH is lower than the isoelectric point (pI) of the protein, & will bind to an anion exchange resin if the pH is higher than the pI.
| Type of exchanger | Functional group | Common name |
| Weak cation exchanger | carboxymethyl -O-CH2-COO- | CM |
| Strong cation exchanger | sulfopropyl -O-CH2-CHOH-CH2-O-CH2-CH2-CH2SO3– | SP |
| Weak anion exchanger | diethylaminoethyl -O-CH2-CH2-N+H(CH2-CH3)2 | DE |
| Strong anion exchanger | quaternary amine -O-CH2-CHOH-CH2-O-CH2-CHOH-CH2-N2(CH3)3 | QAE |
Negative resin
 |
Cation-exchangeLigands: |
Positive resin
 |
Anion-exchangeLigands: |
How IEX works?
IEX is based on electrostatic interactions between a charged molecule & a resin charged opposingly. The resin and the protein must be of opposed charges and the protein must be totaly charged. For this, the pH of the solution must differ from the protein pI of at least 2 pH units.
The pl of the protein (pH where the protein net charge is zero) indicate the pH to be for an excellent molecule adsorption on the support.
Technical tip |
 |
| The electrostatic interactions, repulsions, number & repartition of the charge on the protein surface are responsible of the link. Not the net charges. |
Cation exchange resin |
||
| Acidic pH < to the pI of the protein, interactions preferred with cation exchange resin (The protein is positively charge) | 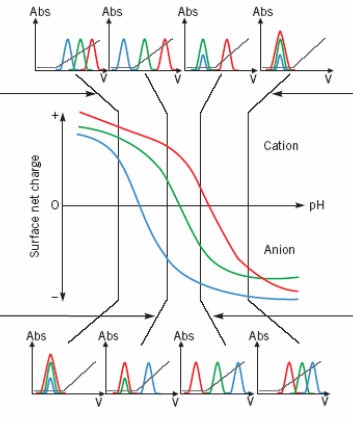 |
Basic pH > to the pI of the protein, interactions preferred with anion exchange resin (The protein is negatively charged) |
| pH less acidic. A negatively charged protein (blue) interact with an anionic resin and can be isolated from the others. Alternatively other proteins can be isolated with a cationic resin when the blue protein is unretained. | pH less basic. A positively charged protein (red) interact with a cationic resin & can be isolated from the others. Alternatively other proteins can be isolated with an anionic resin when the red protein is unretained. | |
Anion exchange resin |
||
Which parameters can be modified to improve a purification?
IEX needs optimisation of binding & elution steps to get a very high purity with high yield.
We can play with the salt concentration and the pH.
| Method | Use |
| Linear salt Gradient | Common |
| Step salt Gradient | Common |
| Isocratic salt gradient | Rare, can give excellent separations |
| Linear pH Gradient | Rare |
| Step pH Gradient | Common |
| Salt & pH Gradient | Common |
A long gradient with low slope increase the resolution
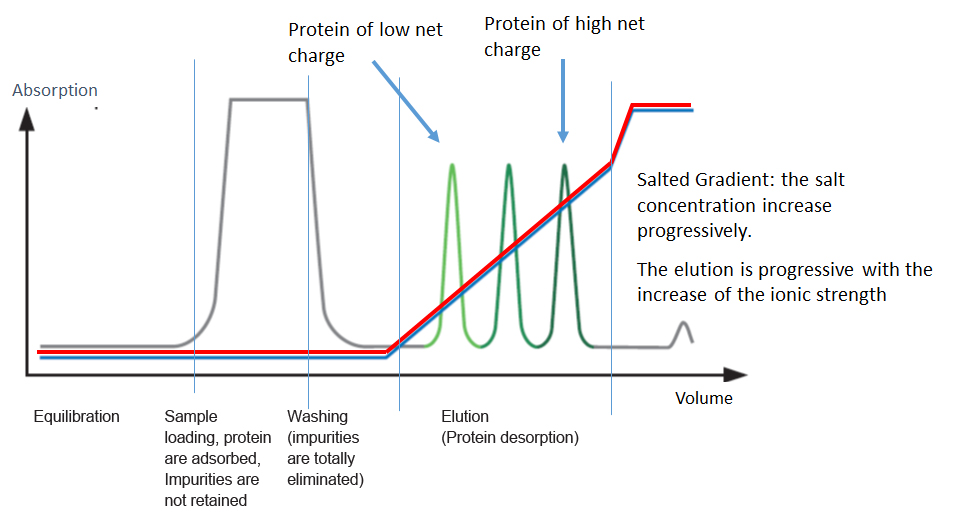
Selectivity & elution |
 |
Linear gradient |
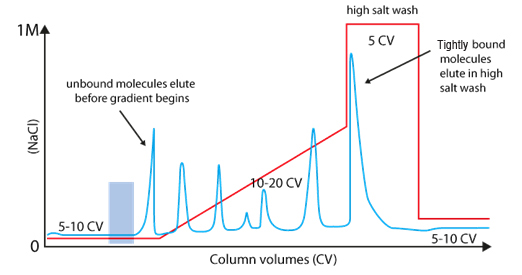 |
Step gradient |
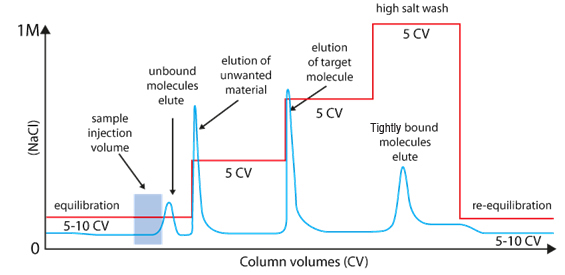 |
Application
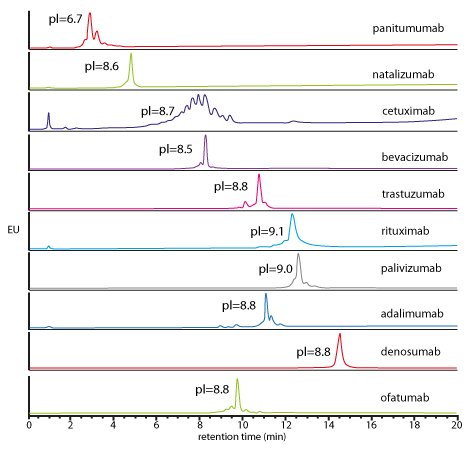
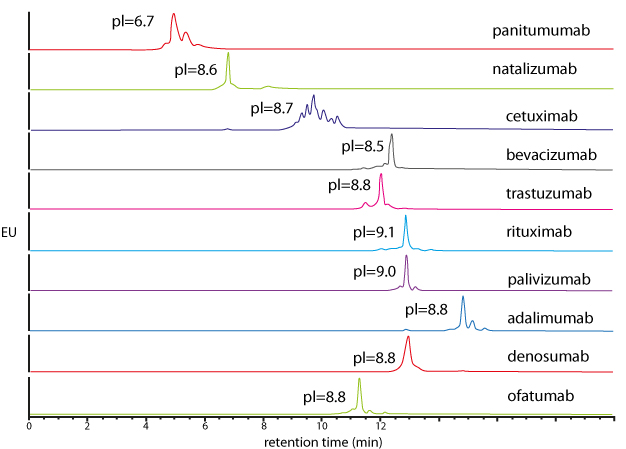
| Generic salt-gradient method for the caracterisation of 10 intact mAbs | Generic pH-gradient method for the caracterisation of 10 intact mAbs |
| Elution of all mAbs (pI between 6.7 and 9.1), using generic salt gradient IEX conditions. Possibility to quantify the mAbs heterogeneity & amount of acidic/basic variantsS. Fekete et al., J. Pharm. Biomed. Anal., 2015, 102, 33-44 |
Similar performance in salt and pH gradient. Interest for pH gradient IEX, mostly in the pharmaceutical industry, to avoid buffer preparation. |
 |
 |
Know more:
- Contact us: interfine@interchim.com
- Visit our website: www.interchim.com
- Discover our product range dedicated to analytical sciences & chromatography
- Discover all our purification systems