Senescence is a cellular state characterized by the cessation of cell division. It serves as a protective mechanism to prevent the propagation of damaged cells, thereby reducing the risk of cancer. However, senescent cells can also contribute to aging and various diseases through the senescence-associated secretory phenotype (SASP), which involves the secretion of inflammatory cytokines, growth factors, and proteases.
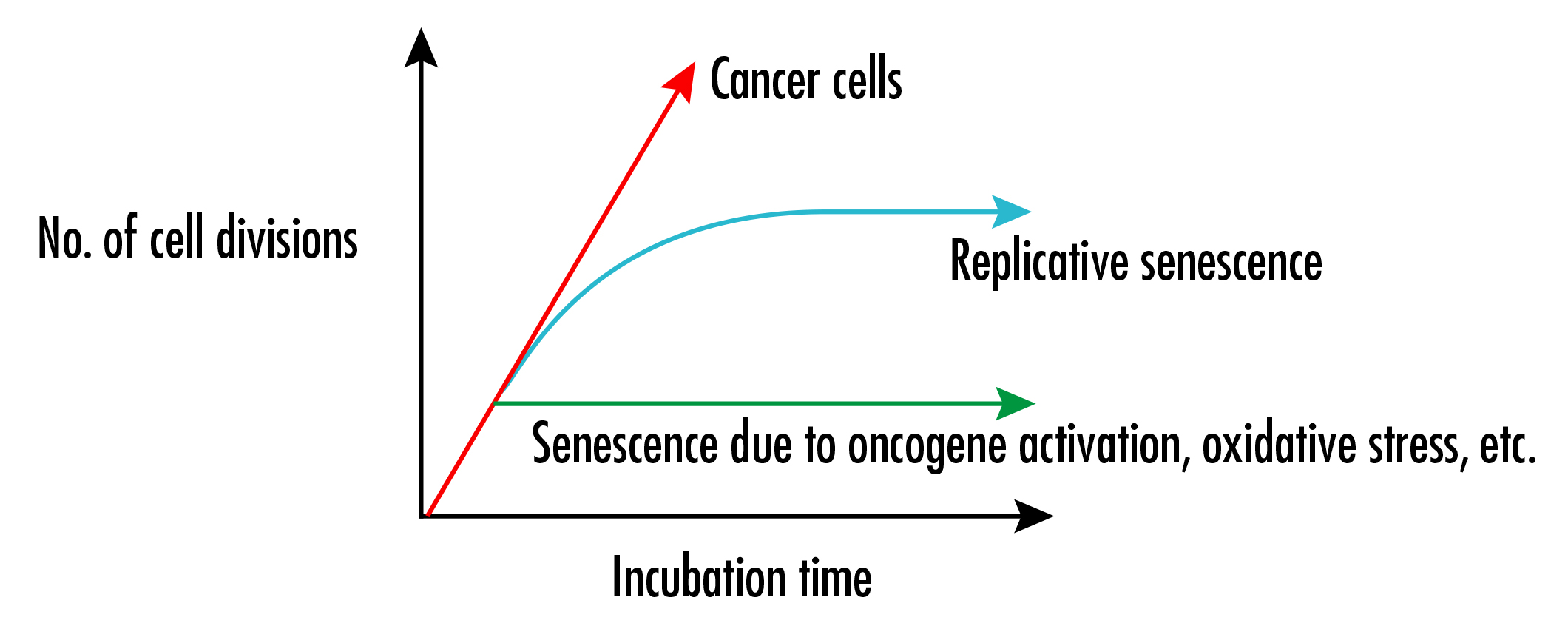
It is clear that cellular senescence is caused not only by the shortening of telomere length, but also by external factors such as oncogene activation and oxidative stress.
Mechanisms of Senescence:
The induction of senescence is regulated by several factors, including tumor suppressor genes, DNA damage response (DDR) pathways, and various signaling molecules. Key players in this process include the p53 and p16INK4a pathways, which respond to cellular stress and damage by initiating senescence.
Senescence-Associated Secretory Phenotype (SASP):
SASP is a hallmark of senescent cells, characterized by the secretion of a variety of bioactive molecules that can alter the tissue microenvironment. This phenotype is regulated by transcription factors such as NF-κB and can have both beneficial and detrimental effects on tissue homeostasis and function.
Mitochondrial and Lysosomal Regulation:
Recent studies have highlighted the importance of mitochondrial and lysosomal function in the regulation of senescence. HKDC1, a protein involved in glycolysis, is essential for maintaining mitochondrial and lysosomal homeostasis. Its absence can lead to cellular senescence and the accumulation of damaged organelles (Mengying Cui, et. al., PNAS, 2023).
Iron Accumulation and Senescence:
Iron metabolism plays a significant role in the regulation of senescence. Vascular and hemolytic injuries can lead to iron accumulation, which promotes fibrosis and the SASP. Senescent cells tend to accumulate iron, particularly within lysosomes, fueling the generation of reactive oxygen species (ROS) and contributing to cellular damage (Mate Maus, et. al., Nature, 2023).
Microautophagy and Lysosomal Repair:
Microautophagy, a process regulated by STK38 and GABARAPs, is crucial for the repair of damaged lysosomes and the prevention of aging. The depletion of these regulators accelerates cellular senescence and shortens lifespan, highlighting the importance of autophagic processes in maintaining cellular health (Monami Ogura, et. al., EMBP Reports, 2023).
Cellular senescence is a double-edged sword, providing protection against cancer while contributing to aging and disease through the SASP. Targeting senescence and its associated pathways offers potential therapeutic strategies for age-related diseases and cancer. Modulating the activity of key regulators such as HKDC1, iron metabolism, and autophagic processes could mitigate the detrimental effects of senescence and improve healthspan. Understanding the mechanisms underlying senescence and developing targeted interventions could pave the way for novel therapies to combat age-related pathologies.
Related Techniques
- Cellular senescence detection: live-cell imaging or flow cytometry / microplate reader / tissue samples
- First-time autophagy research: autophagic Flux Assay Kit
- Autophagy detection: DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
- Lysosomal function: Lysosomal Acidic pH Detection Kit-Green /Red and Green/Deep Red
- Ferrous ion (Fe2+) detection: FerroOrange (intracellular), Mito-FerroGreen (mitochondria)
- Mitochondrial superoxide detection: MitoBright ROS Deep Red – Mitochondrial Superoxide Detection
- Oxygen consumption rate assay: Extracellular OCR Plate Assay Kit
Learn More:
- For further information, download the associated brochures:
- Contact for any technical, application or commercial support:
- Phone: +33 4 70 03 73 06
- Email: consumables.eu@advion-interchim.com
- Follow our news on LinkedIn