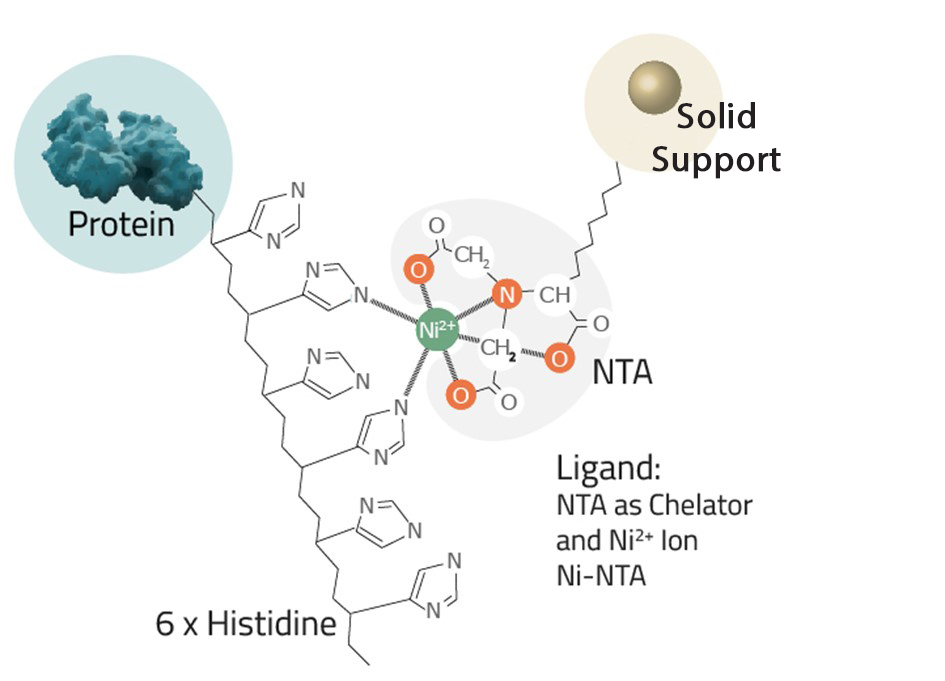
Poly-histidine (His)-tagged proteins are commonly used in recombinant protein expression and purification. The His-tag offers advantages such as small size, high affinity for binding to IMAC (Immobilized Metal Affinity Chromatography) resins, and one-step purification. However, there are instances when a His-tagged protein fails to bind to the affinity column, leading to frustration. In this article, we discuss troubleshooting steps to address this issue.
Common Challenges and Solutions
1. Solubilization Buffer Optimization
Problem: Ni-NTA resins are sensitive to reducing agents (e.g., DTT) and chelating agents (e.g., EDTA or EGTA). These substances can affect the binding capacity of IMAC resins.
Solution:
Ensure that solubilization buffers do not contain reducing or chelating agents.
Maintain an imidazole concentration of 15-25 mM in the binding buffer to prevent non-specific protein binding.
Adjust the pH of the buffer close to the protein’s isoelectric point.
2. Fresh and Functional Affinity Resin
Problem: Using old or compromised Ni-NTA resin can lead to poor binding.
Solution:
Always use fresh Ni-NTA resin, especially when purifying a novel protein for the first time.
Perform batch binding with a control His-tagged protein (previously purified using IMAC) alongside your protein of interest. If the control protein binds but your protein does not, investigate further.
3. Hidden His-Tag
Problem: Some proteins bury the His-tag within their folded native structure, rendering it inaccessible for metal coordination.
Solution:
Test binding in the presence of denaturing agents (e.g., urea or guanidinium chloride). If the His-tagged fusion protein binds under denaturing conditions, hidden tag folding may be the issue.
Options:
Purify the protein under denaturing conditions and then refold it.
Add a flexible linker (e.g., poly-serine or poly-glycine) to separate the tag from the fusion protein.
Try placing the His tag at the opposite terminus of the construct.
4. Non-Optimal Binding Buffer
Problem: Buffer composition affects His-tag binding.
Solution:
Pay attention to imidazole concentration and pH in the binding buffer.
Optimize buffer components to enhance binding efficiency.
Alternatives tags:
Glutathione-S-Transferase (GST) Tag: for purification and pull-down assays
Biotin Tag: for immobilization or detection
Maltose-Binding Protein (MBP) Tag: enhances solubility – for affinity purification and protein-protein interaction studies
Strep-Tag® II: high-affinity binding to streptavidin – for purification and detection applications
FLAG™ (DYKDDDDK) Tag: for detection, immunoprecipitation, and Western blotting
SUMO Tag: removable – to produce native N-term protein
Myc Tag: removable – for purification, detection and crystallization
Fc Tag: IgG constant domain – for ELISA detection, affinity purification and increase expression
Learn More:
- Discover the catalogs:
- Contact for any technical, application or commercial support:
- Phone: +33 4 70 03 73 06
- Email: consumables.eu@advion-interchim.com
- Follow our news on LinkedIn